Thrombotic microangiopathy associated with interferon beta
- PMID: 24670186
- PMCID: PMC4066182
- DOI: 10.1056/NEJMc1316118
Thrombotic microangiopathy associated with interferon beta
Figures
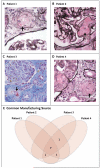
References
-
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–504. - PubMed
- Lancet. 1999;353:678. Erratum.
-
- Recombinant interferon-beta: thrombotic microangiopathy [December 2013];Drug Safety Update. :S3. http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON355481.
-
- Giovannoni G, Barbarash O, Casset-Semanaz F, et al. Safety and immunogenicity of a new formulation of interferon beta-1a (Rebif New Formulation) in a Phase IIIb study in patients with relapsing multiple sclerosis: 96-week results. Mult Scler. 2009;15:219–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
