Novel neuromuscular electrical stimulation system for treatment of dysphagia after brain injury
- PMID: 24670314
- PMCID: PMC4533457
- DOI: 10.2176/nmc.oa.2013-0341
Novel neuromuscular electrical stimulation system for treatment of dysphagia after brain injury
Abstract
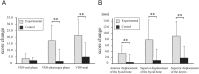
The purpose of this study was to compare the effects of a novel neuromuscular electrical stimulation (NMES) to the effects of conventional treatment in patients with dysphagia after brain injury. In total, 26 patients were non-randomly divided into an experimental group (n = 12) and a control group (n = 14). The experimental group received NMES intervention followed by conventional treatment, including thermaltactile stimulation with intensive repetition of a dry-swallow task. The control group received conventional treatment without NMES. NMES at a fixed pulse duration of 50 μs and a frequency of 50 Hz was delivered over the skin areas above the motor point of the target muscles, i.e., the bilateral geniohyoid, mylohyoid/anterior belly of the digastric, and thyrohyoid muscles, using a high-voltage pulsed-current device. The two groups received 40-min treatments once a day, 5 days per week, for 8 weeks. Outcome, assessed before and 8 weeks after treatment, was evaluated with regard to the videofluoroscopic dysphagia scale (VDS), the anterior and superior displacement of the hyoid bone and larynx, and the functional oral intake scale. Both groups exhibited improvement, but the experimental group exhibited more significant improvement in the displacement of the hyoid bone and larynx, VDS-total score, and VDS-pharyngeal score than the control group did. The results suggest that NMES combined with conventional treatment is superior to conventional treatment alone in patients with dysphagia following treatment for brain injury. Further investigations are necessary to examine the effects of NMES in patients with more varied types of diseases.
Conflict of interest statement
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices in the article.
Figures



References
-
- Blumenfeld L, Hahn Y, Lepage A, Leonard R, Belafsky PC: Transcutaneous electrical stimulation versus traditional dysphagia therapy: a nonconcurrent cohort study. Otolaryngol Head Neck Surg 135: 754– 757, 2006. - PubMed
-
- Carnaby-Mann GD, Crary MA: Adjunctive neuromuscular electrical stimulation for treatment-refractory dysphagia. Ann Otol Rhinol Laryngol 117: 279– 287, 2008. - PubMed
-
- Freed ML, Freed L, Chatburn RL, Christian M: Electrical stimulation for swallowing disorders caused by stroke. Respir Care 46: 466– 474, 2001. - PubMed
-
- Kiger M, Brown CS, Watkins L: Dysphagia management: an analysis of patient outcomes using VitalStim therapy compared to traditional swallow therapy. Dysphagia 21: 243– 253, 2006. - PubMed
-
- Suiter DM, Leder SB, Ruark JL: Effects of neuromuscular electrical stimulation on submental muscle activity. Dysphagia 21: 56– 60, 2006. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

