Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity
- PMID: 24670648
- PMCID: PMC4932833
- DOI: 10.1038/nature13158
Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity
Abstract
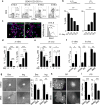
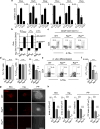
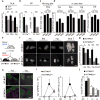
The impact of nutritional status during fetal life on the overall health of adults has been recognized; however, dietary effects on the developing immune system are largely unknown. Development of secondary lymphoid organs occurs during embryogenesis and is considered to be developmentally programmed. Secondary lymphoid organ formation depends on a subset of type 3 innate lymphoid cells (ILC3) named lymphoid tissue inducer (LTi) cells. Here we show that mouse fetal ILC3s are controlled by cell-autonomous retinoic acid (RA) signalling in utero, which pre-sets the immune fitness in adulthood. We found that embryonic lymphoid organs contain ILC progenitors that differentiate locally into mature LTi cells. Local LTi cell differentiation was controlled by maternal retinoid intake and fetal RA signalling acting in a haematopoietic cell-autonomous manner. RA controlled LTi cell maturation upstream of the transcription factor RORγt. Accordingly, enforced expression of Rorgt restored maturation of LTi cells with impaired RA signalling, whereas RA receptors directly regulated the Rorgt locus. Finally, we established that maternal levels of dietary retinoids control the size of secondary lymphoid organs and the efficiency of immune responses in the adult offspring. Our results reveal a molecular link between maternal nutrients and the formation of immune structures required for resistance to infection in the offspring.
Conflict of interest statement
Figures
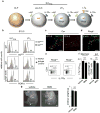


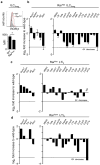
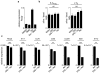

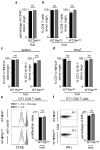



Comment in
-
Immunology: A is for immunity.Nature. 2014 Apr 3;508(7494):47-8. doi: 10.1038/nature13216. Epub 2014 Mar 19. Nature. 2014. PMID: 24670655 No abstract available.
-
Development: we are what mum eats!Nat Rev Immunol. 2014 May;14(5):284-5. doi: 10.1038/nri3672. Epub 2014 Apr 22. Nat Rev Immunol. 2014. PMID: 24751954 No abstract available.
References
-
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. - PubMed
-
- van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. - PubMed
-
- Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity. 1997;7:493–504. - PubMed
-
- Eberl G, et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

