Structure of a type IV secretion system
- PMID: 24670658
- PMCID: PMC3998870
- DOI: 10.1038/nature13081
Structure of a type IV secretion system
Abstract
Bacterial type IV secretion systems translocate virulence factors into eukaryotic cells, distribute genetic material between bacteria and have shown potential as a tool for the genetic modification of human cells. Given the complex choreography of the substrate through the secretion apparatus, the molecular mechanism of the type IV secretion system has proved difficult to dissect in the absence of structural data for the entire machinery. Here we use electron microscopy to reconstruct the type IV secretion system encoded by the Escherichia coli R388 conjugative plasmid. We show that eight proteins assemble in an intricate stoichiometric relationship to form an approximately 3 megadalton nanomachine that spans the entire cell envelope. The structure comprises an outer membrane-associated core complex connected by a central stalk to a substantial inner membrane complex that is dominated by a battery of 12 VirB4 ATPase subunits organized as side-by-side hexameric barrels. Our results show a secretion system with markedly different architecture, and consequently mechanism, to other known bacterial secretion systems.
Figures

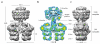


Comment in
-
Structural biology: solving the T4SS structural mystery.Nat Rev Microbiol. 2014 May;12(5):312. doi: 10.1038/nrmicro3254. Epub 2014 Apr 1. Nat Rev Microbiol. 2014. PMID: 24686411 No abstract available.
References
-
- Schröder G, Lanka E. The mating pair formation system of conjugative plasmids - A versatile secretion machinery for transfer of proteins and DNA. Plasmid. 2005;54:1–25. - PubMed
-
- Johnson TL, Abendroth J, Hol WG, Sandkvist M. Type II secretion: from structure to function. FEMS microbiology letters. 2006;255:175–186. - PubMed
Supplementary references
-
- Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. Journal of computational chemistry. 2004;25:1605–1612. - PubMed
-
- De Carlo S, Boisset N, Hoenger A. High-resolution single-particle 3D analysis on GroEL prepared by cryo-negative staining. Micron. 2008;39:934–943. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

