Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss
- PMID: 24670684
- PMCID: PMC4090608
- DOI: 10.1126/scitranslmed.3007696
Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss
Abstract
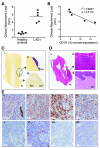
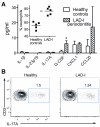
Leukocyte adhesion deficiency type I (LAD-I), a disease syndrome associated with frequent microbial infections, is caused by mutations on the CD18 subunit of β₂ integrins. LAD-I is invariably associated with severe periodontal bone loss, which historically has been attributed to the lack of neutrophil surveillance of the periodontal infection. We provide an alternative mechanism by showing that the cytokine interleukin-17 (IL-17) plays a major role in the oral pathology of LAD-I. Defective neutrophil recruitment in LAD-I patients or in LFA-1 (CD11a/CD18)-deficient mice--which exhibit the LAD-I periodontal phenotype--was associated with excessive production of predominantly T cell-derived IL-17 in the periodontal tissue, although innate lymphoid cells also contributed to pathological IL-17 elevation in the LFA-1-deficient mice. Local treatment with antibodies to IL-17 or IL-23 in LFA-1-deficient mice not only blocked inflammatory periodontal bone loss but also caused a reduction in the total bacterial burden, suggesting that the IL-17-driven pathogenesis of LAD-I periodontitis leads to dysbiosis. Therefore, our findings support an IL-17-targeted therapy for periodontitis in LAD-I patients.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

