Comparative effectiveness of sphincter-sparing surgery versus abdominoperineal resection in rectal cancer: patient-reported outcomes in National Surgical Adjuvant Breast and Bowel Project randomized trial R-04
- PMID: 24670844
- PMCID: PMC4379325
- DOI: 10.1097/SLA.0000000000000594
Comparative effectiveness of sphincter-sparing surgery versus abdominoperineal resection in rectal cancer: patient-reported outcomes in National Surgical Adjuvant Breast and Bowel Project randomized trial R-04
Abstract
Objective: National Surgical Adjuvant Breast and Bowel Project (NSABP) R-04 was a randomized controlled trial of neoadjuvant chemoradiotherapy in patients with resectable stage II-III rectal cancer. We hypothesized that patients who underwent abdominoperineal resection (APR) would have a poorer quality of life than those who underwent sphincter-sparing surgery (SSS).
Methods: To obtain patient-reported outcomes (PROs) we administered two symptom scales at baseline and 1 year postoperatively: the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) and the European Organization for the Research and Treatment of Cancer module for patients with Colorectal Cancer Quality of Life Questionnaire (EORTC QLQ-CR38). Scoring was stratified by nonrandomly assigned definitive surgery (APR vs SSS). Analyses controlled for baseline scores and stratification factors: age, sex, stage, intended surgery, and randomly assigned chemoradiotherapy.
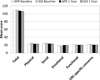
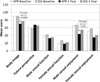
Results: Of 1,608 randomly assigned patients, 987 had data for planned analyses; 62% underwent SSS; 38% underwent APR. FACT-C total and subscale scores were not statistically different by surgery at 1 year. For the EORTC QLQ-CR38 functional scales, APR patients reported worse body image (70.3 vs 77.0, P = 0.0005) at 1 year than did SSS patients. Males undergoing APR reported worse sexual enjoyment (43.7 vs 54.7, P = 0.02) at 1 year than did those undergoing SSS. For the EORTC QLQ-CR38 symptom scale scores, APR patients reported worse micturition symptoms than the SSS group at 1 year (26.9 vs 21.5, P = 0.03). SSS patients reported worse gastrointestinal tract symptoms than did the APR patients (18.9 vs 15.2, P < 0.0001), as well as weight loss (10.1 vs 6.0, P = 0.002).
Conclusions: Symptoms and functional problems were detected at 1 year by EORTC QLQ-CR38, reflecting different symptom profiles in patients who underwent APR than those who underwent SSS. Information from these PROs may be useful in counseling patients anticipating surgery for rectal cancer.
Trial registration: ClinicalTrials.gov NCT00058474.
Conflict of interest statement
All authors declare support from NCI-U10-CA-12027, U10-CA-37377, U10-CA-69974, U10-CA-69651, and U10-CA-21661, by Roche Laboratories Inc., a full member of the Roche Group of companies, and by Sanofi-Synthelabo Inc.
Patricia A. Ganz, MD – declares support for travel for NSABP group meetings. Greg Yothers, PhD – declares support for provision of writing assistance, medicines, equipment, or administrative support (Past).
All other authors declare no other potential conflict(s) of interest.
Figures


References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2006.
-
- Pucciarelli S, Del Bianco P, Efficace F, et al. Patient-reported outcomes after neoadjuvant chemoradiotherapy for rectal cancer: a multicenter prospective observational study. Ann Surg. 2011;253:71–77. - PubMed
-
- How P, Stelzner S, Branagan G, et al. Comparative quality of life in patients following abdominoperineal excision and low anterior resection for low rectal cancer. Dis Colon Rectum. 2012;55:400–406. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- UG1 CA189867/CA/NCI NIH HHS/United States
- U10-CA-37377/CA/NCI NIH HHS/United States
- U10-CA-21661/CA/NCI NIH HHS/United States
- U10 CA012027/CA/NCI NIH HHS/United States
- U10 CA069651/CA/NCI NIH HHS/United States
- U10 CA021661/CA/NCI NIH HHS/United States
- U10 CA069974/CA/NCI NIH HHS/United States
- U10 CA180822/CA/NCI NIH HHS/United States
- U10 CA037377/CA/NCI NIH HHS/United States
- U10 CA180868/CA/NCI NIH HHS/United States
- U10-CA-69651/CA/NCI NIH HHS/United States
- U10-CA-69974/CA/NCI NIH HHS/United States
- U10-CA-12027/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

