Hypoxia-induced changes in Ca(2+) mobilization and protein phosphorylation implicated in impaired wound healing
- PMID: 24671101
- PMCID: PMC4024710
- DOI: 10.1152/ajpcell.00110.2013
Hypoxia-induced changes in Ca(2+) mobilization and protein phosphorylation implicated in impaired wound healing
Abstract
The process of wound healing must be tightly regulated to achieve successful restoration of injured tissue. Previously, we demonstrated that when corneal epithelium is injured, nucleotides and neuronal factors are released to the extracellular milieu, generating a Ca(2+) wave from the origin of the wound to neighboring cells. In the present study we sought to determine how the communication between epithelial cells in the presence or absence of neuronal wound media is affected by hypoxia. A signal-sorting algorithm was developed to determine the dynamics of Ca(2+) signaling between neuronal and epithelial cells. The cross talk between activated corneal epithelial cells in response to neuronal wound media demonstrated that injury-induced Ca(2+) dynamic patterns were altered in response to decreased O2 levels. These alterations were associated with an overall decrease in ATP and changes in purinergic receptor-mediated Ca(2+) mobilization and localization of N-methyl-d-aspartate receptors. In addition, we used the cornea in an organ culture wound model to examine how hypoxia impedes reepithelialization after injury. There was a change in the recruitment of paxillin to the cell membrane and deposition of fibronectin along the basal lamina, both factors in cell migration. Our results provide evidence that complex Ca(2+)-mediated signaling occurs between sensory neurons and epithelial cells after injury and is critical to wound healing. Information revealed by these studies will contribute to an enhanced understanding of wound repair under compromised conditions and provide insight into ways to effectively stimulate proper epithelial repair.
Keywords: cell communication; hypoxia; imaging; wound healing.
Copyright © 2014 the American Physiological Society.
Figures


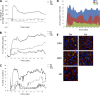



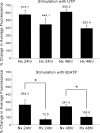
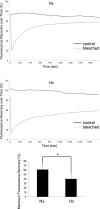
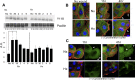

References
-
- Araki K, Ohashi Y, Kinoshita S, Hayashi K, Kuwayama Y, Tano Y. Epithelial wound healing in the denervated cornea. Curr Eye Res 13: 203–211, 1994 - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. Signal transduction. The calcium entry pas de deux. Science 287: 1604–1605, 2000 - PubMed
-
- Besshoh S, Chen S, Brown IR, Gurd JW. Developmental changes in the association of NMDA receptors with lipid rafts. J Neurosci Res 85: 1876–1883, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

