Beta-actin deficiency with oxidative posttranslational modifications in Rett syndrome erythrocytes: insights into an altered cytoskeletal organization
- PMID: 24671107
- PMCID: PMC3966888
- DOI: 10.1371/journal.pone.0093181
Beta-actin deficiency with oxidative posttranslational modifications in Rett syndrome erythrocytes: insights into an altered cytoskeletal organization
Abstract
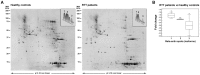
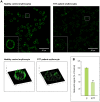
Beta-actin, a critical player in cellular functions ranging from cell motility and the maintenance of cell shape to transcription regulation, was evaluated in the erythrocyte membranes from patients with typical Rett syndrome (RTT) and methyl CpG binding protein 2 (MECP2) gene mutations. RTT, affecting almost exclusively females with an average frequency of 1∶10,000 female live births, is considered the second commonest cause of severe cognitive impairment in the female gender. Evaluation of beta-actin was carried out in a comparative cohort study on red blood cells (RBCs), drawn from healthy control subjects and RTT patients using mass spectrometry-based quantitative analysis. We observed a decreased expression of the beta-actin isoforms (relative fold changes for spots 1, 2 and 3: -1.82±0.15, -2.15±0.06, and -2.59±0.48, respectively) in pathological RBCs. The results were validated by western blotting and immunofluorescence microscopy. In addition, beta-actin from RTT patients also showed a dramatic increase in oxidative posttranslational modifications (PTMs) as the result of its binding with the lipid peroxidation product 4-hydroxy-2-nonenal (4-HNE). Our findings demonstrate, for the first time, a beta-actin down-regulation and oxidative PTMs for RBCs of RTT patients, thus indicating an altered cytoskeletal organization.
Conflict of interest statement
Figures




References
-
- Herman IM (1993) Actin isoforms. Curr Opin Cell Biol 5: 48–55. - PubMed
-
- Kaufmann WE, Moser HW (2000) Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex 10: 981–91. - PubMed
-
- Kunze D, Rüstow B (1993) Pathobiochemical aspects of cytoskeleton components. Eur J Clin Chem Clin Biochem 31: 477–89. - PubMed
-
- Kaufmann WE, MacDonald SM, Altamura CR (2000) Dendritic cytoskeletal protein expression in mental retardation: an immunohistochemical study of the neocortex in Rett Syndrome. Cereb Cortex 10: 992–1004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

