A novel oncolytic herpes simplex virus type 2 has potent anti-tumor activity
- PMID: 24671154
- PMCID: PMC3966855
- DOI: 10.1371/journal.pone.0093103
A novel oncolytic herpes simplex virus type 2 has potent anti-tumor activity
Abstract
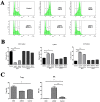
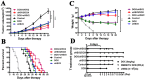
Oncolytic viruses are promising treatments for many kinds of solid tumors. In this study, we constructed a novel oncolytic herpes simplex virus type 2: oHSV2. We investigated the cytopathic effects of oHSV2 in vitro and tested its antitumor efficacy in a 4T1 breast cancer model. We compared its effect on the cell cycle and its immunologic impact with the traditional chemotherapeutic agent doxorubicin. In vitro data showed that oHSV2 infected most of the human and murine tumor cell lines and was highly oncolytic. oHSV2 infected and killed 4T1 tumor cells independent of their cell cycle phase, whereas doxorubicin mainly blocked cells that were in S and G2/M phase. In vivo study showed that both oHSV2 and doxorubicin had an antitumor effect, though the former was less toxic. oHSV2 treatment alone not only slowed down the growth of tumors without causing weight loss but also induced an elevation of NK cells and mild decrease of Tregs in spleen. In addition, combination therapy of doxorubicin followed by oHSV2 increased survival with weight loss than oHSV2 alone. The data showed that the oncolytic activity of oHSV2 was similar to oHSV1 in cell lines examined and in vivo. Therefore, we concluded that our virus is a safe and effective therapeutic agent for 4T1 breast cancer and that the sequential use of doxorubicin followed by oHSV2 could improve antitumor activity without enhancing doxorubicin's toxicity.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

