Redistribution of flexibility in stabilizing antibody fragment mutants follows Le Châtelier's principle
- PMID: 24671209
- PMCID: PMC3966838
- DOI: 10.1371/journal.pone.0092870
Redistribution of flexibility in stabilizing antibody fragment mutants follows Le Châtelier's principle
Abstract
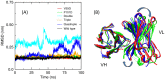
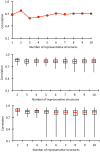
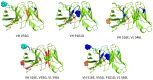
Le Châtelier's principle is the cornerstone of our understanding of chemical equilibria. When a system at equilibrium undergoes a change in concentration or thermodynamic state (i.e., temperature, pressure, etc.), La Châtelier's principle states that an equilibrium shift will occur to offset the perturbation and a new equilibrium is established. We demonstrate that the effects of stabilizing mutations on the rigidity ⇔ flexibility equilibrium within the native state ensemble manifest themselves through enthalpy-entropy compensation as the protein structure adjusts to restore the global balance between the two. Specifically, we characterize the effects of mutation to single chain fragments of the anti-lymphotoxin-β receptor antibody using a computational Distance Constraint Model. Statistically significant changes in the distribution of both rigidity and flexibility within the molecular structure is typically observed, where the local perturbations often lead to distal shifts in flexibility and rigidity profiles. Nevertheless, the net gain or loss in flexibility of individual mutants can be skewed. Despite all mutants being exclusively stabilizing in this dataset, increased flexibility is slightly more common than increased rigidity. Mechanistically the redistribution of flexibility is largely controlled by changes in the H-bond network. For example, a stabilizing mutation can induce an increase in rigidity locally due to the formation of new H-bonds, and simultaneously break H-bonds elsewhere leading to increased flexibility distant from the mutation site via Le Châtelier. Increased flexibility within the VH β4/β5 loop is a noteworthy illustration of this long-range effect.
Conflict of interest statement
Figures
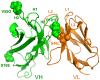




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

