Lysine 27 ubiquitination of the mitochondrial transport protein Miro is dependent on serine 65 of the Parkin ubiquitin ligase
- PMID: 24671417
- PMCID: PMC4031514
- DOI: 10.1074/jbc.M114.563031
Lysine 27 ubiquitination of the mitochondrial transport protein Miro is dependent on serine 65 of the Parkin ubiquitin ligase
Abstract
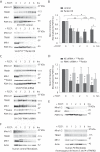
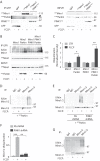
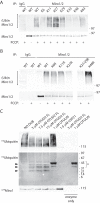
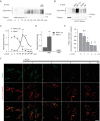
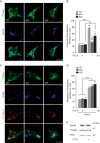
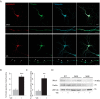
Mitochondrial transport plays an important role in matching mitochondrial distribution to localized energy production and calcium buffering requirements. Here, we demonstrate that Miro1, an outer mitochondrial membrane (OMM) protein crucial for the regulation of mitochondrial trafficking and distribution, is a substrate of the PINK1/Parkin mitochondrial quality control system in human dopaminergic neuroblastoma cells. Moreover, Miro1 turnover on damaged mitochondria is altered in Parkinson disease (PD) patient-derived fibroblasts containing a pathogenic mutation in the PARK2 gene (encoding Parkin). By analyzing the kinetics of Miro1 ubiquitination, we further demonstrate that mitochondrial damage triggers rapid (within minutes) and persistent Lys-27-type ubiquitination of Miro1 on the OMM, dependent on PINK1 and Parkin. Proteasomal degradation of Miro1 is then seen on a slower time scale, within 2-3 h of the onset of ubiquitination. We find Miro ubiquitination in dopaminergic neuroblastoma cells is independent of Miro1 phosphorylation at Ser-156 but is dependent on the recently identified Ser-65 residue within Parkin that is phosphorylated by PINK1. Interestingly, we find that Miro1 can stabilize phospho-mutant versions of Parkin on the OMM, suggesting that Miro is also part of a Parkin receptor complex. Moreover, we demonstrate that Ser-65 in Parkin is critical for regulating Miro levels upon mitochondrial damage in rodent cortical neurons. Our results provide new insights into the ubiquitination-dependent regulation of the Miro-mediated mitochondrial transport machinery by PINK1/Parkin and also suggest that disruption of this regulation may be implicated in Parkinson disease pathogenesis.
Keywords: Mitochondrial Transport; Mitophagy; Parkin; Parkinson Disease; Pink1; Ubiquitination.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Fransson A., Ruusala A., Aspenström P. (2003) Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J. Biol. Chem. 278, 6495–6502 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

