Sensory convergence in the parieto-insular vestibular cortex
- PMID: 24671533
- PMCID: PMC4044443
- DOI: 10.1152/jn.00731.2013
Sensory convergence in the parieto-insular vestibular cortex
Abstract
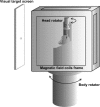
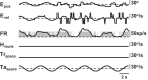
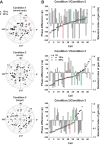
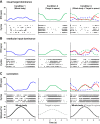
Vestibular signals are pervasive throughout the central nervous system, including the cortex, where they likely play different roles than they do in the better studied brainstem. Little is known about the parieto-insular vestibular cortex (PIVC), an area of the cortex with prominent vestibular inputs. Neural activity was recorded in the PIVC of rhesus macaques during combinations of head, body, and visual target rotations. Activity of many PIVC neurons was correlated with the motion of the head in space (vestibular), the twist of the neck (proprioceptive), and the motion of a visual target, but was not associated with eye movement. PIVC neurons responded most commonly to more than one stimulus, and responses to combined movements could often be approximated by a combination of the individual sensitivities to head, neck, and target motion. The pattern of visual, vestibular, and somatic sensitivities on PIVC neurons displayed a continuous range, with some cells strongly responding to one or two of the stimulus modalities while other cells responded to any type of motion equivalently. The PIVC contains multisensory convergence of self-motion cues with external visual object motion information, such that neurons do not represent a specific transformation of any one sensory input. Instead, the PIVC neuron population may define the movement of head, body, and external visual objects in space and relative to one another. This comparison of self and external movement is consistent with insular cortex functions related to monitoring and explains many disparate findings of previous studies.
Keywords: insula; multisensory; self-motion; sensory integration; spatial orientation.
Copyright © 2014 the American Physiological Society.
Figures






References
-
- Abreu AM, Macaluso E, Azevedo RT, Cesari P, Urgesi C, Aglioti SM. Action anticipation beyond the action observation network: a functional magnetic resonance imaging study in expert basketball players. Eur J Neurosci 35: 1646–1654, 2012 - PubMed
-
- Akbarian S, Berndl K, Grusser OJ, Guldin W, Pause M, Schreiter U. Responses of single neurons in the parietoinsular vestibular cortex of primates. Ann NY Acad Sci 545: 187–202, 1988 - PubMed
-
- Akbarian S, Grusser OJ, Guldin WO. Corticofugal connections between the cerebral cortex and brainstem vestibular nuclei in the Macaque monkey. J Comp Neurol 339: 421–437, 1994 - PubMed
-
- Alitto HJ, Usrey WM. Corticothalamic feedback and sensory processing. Curr Opin Neurobiol 13: 440–445, 2003 - PubMed
-
- Antal A, Baudewig J, Paulus W, Dechent P. The posterior cingulate cortex and planum temporale/parietal operculum are activated by coherent visual motion. Vis Neurosci 25: 17–26, 2008 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

