Oxidation of methionine residues in polypeptide ions via gas-phase ion/ion chemistry
- PMID: 24671696
- PMCID: PMC4020970
- DOI: 10.1007/s13361-014-0861-8
Oxidation of methionine residues in polypeptide ions via gas-phase ion/ion chemistry
Abstract
The gas-phase oxidation of methionine residues is demonstrated here using ion/ion reactions with periodate anions. Periodate anions are observed to attach in varying degrees to all polypeptide ions irrespective of amino acid composition. Direct proton transfer yielding a charge-reduced peptide ion is also observed. In the case of methionine and, to a much lesser degree, tryptophan-containing peptide ions, collisional activation of the complex ion generated by periodate attachment yields an oxidized peptide product (i.e., [M + H + O](+)), in addition to periodic acid detachment. Detachment of periodic acid takes place exclusively for peptides that do not contain either a methionine or tryptophan side chain. In the case of methionine-containing peptides, the [M + H + O](+) product is observed at a much greater abundance than the proton transfer product (viz., [M + H](+)). Collisional activation of oxidized Met-containing peptides yields a signature loss of 64 Da from the precursor and/or product ions. This unique loss corresponds to the ejection of methanesulfenic acid from the oxidized methionine side chain and is commonly used in solution-phase proteomics studies to determine the presence of oxidized methionine residues. The present work shows that periodate anions can be used to 'label' methionine residues in polypeptides in the gas phase. The selectivity of the periodate anion for the methionine side chain suggests several applications including identification and location of methionine residues in sequencing applications.
Figures

 ) is used to denote the ion that has been subjected to CID.
) is used to denote the ion that has been subjected to CID.
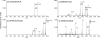
 ) is used to denote the ion that has been subjected to CID.
) is used to denote the ion that has been subjected to CID.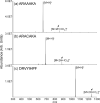



 ) is used to denote the ion that has been subjected to CID.
) is used to denote the ion that has been subjected to CID.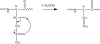
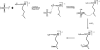
References
-
- Berlett BS, Stadtman ER. Protein Oxidation in Aging, Disease, and Oxidative Stress. J. Biol. Chem. 1997;272:20313–20316. - PubMed
-
- Mulinacci F, Poirier E, Capelle MAH, Gurny R, Arvinte T. Influence of methionine oxidation on the aggregation of recombinant human growth hormone. Eur. J. of Pharm. And Biopharm. 2013;85:42–52. - PubMed
-
- Vogt W. Oxidation of methionyl residues in proteins – tools, targets, and reversal. Free Radical Biology and Medicine. 1995;18:93–105. - PubMed
-
- Paik WK, Kim S. Protein Methylation - Chemical, Enzymological, and Biological Significance. Adv. Enzymol. 1975;42:277–286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

