Deimination of linker histones links neutrophil extracellular trap release with autoantibodies in systemic autoimmunity
- PMID: 24671707
- PMCID: PMC4756806
- DOI: 10.1096/fj.13-247254
Deimination of linker histones links neutrophil extracellular trap release with autoantibodies in systemic autoimmunity
Abstract
Autoantibodies to nuclear antigens arise in human autoimmune diseases, but a unifying pathogenetic mechanism remains elusive. Recently we reported that exposure of neutrophils to inflammatory conditions induces the citrullination of core histones by peptidylarginine deiminase 4 (PAD4) and that patients with autoimmune disorders produce autoantibodies that recognize such citrullinated histones. Here we identify histone H1 as an additional substrate of PAD4, localize H1 within neutrophil extracellular traps, and detect autoantibodies to citrullinated H1 in 6% of sera from patients with systemic lupus erythematosus and Sjögren's syndrome. No preference for deiminated H1 was observed in healthy control sera and sera from patients with scleroderma or rheumatoid arthritis. We map binding to the winged helix of H1 and determine that citrulline 53 represents a key determinant of the autoantibody epitope. In addition, we quantitate RNA for H1 histone subtypes in mature human neutrophils and identify citrulline residues by liquid chromatography and tandem mass spectrometry. Our results indicate that deimination of linker histones generates new autoantibody epitopes with enhanced potential for stimulating autoreactive human B cells.-Dwivedi, N., Neeli, I., Schall, N., Wan, H., Desiderio, D. M., Csernok, E., Thompson, P. R., Dali, H., Briand, J.-P., Muller, S., Radic, M. Deimination of linker histones links neutrophil extracellular trap release with autoantibodies in systemic autoimmunity.
Keywords: autoimmune disorders; chromatin; inflammation; peptidylarginine deiminase.
© FASEB.
Figures
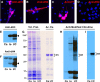




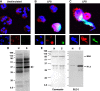
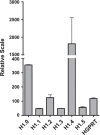
References
-
- Muller S., Dieker J., Tincani A., Meroni P. L. (2008) Pathogenic anti-nucleosome antibodies. Lupus 17, 431–436 - PubMed
-
- Fauchais A. L., Martel C., Gondran G., Lambert M., Launay D., Jauberteau M. O., Hachulla E., Vidal E., Hatron P. Y. (2010) Immunological profile in primary Sjögren syndrome: clinical significance, prognosis and long-term evolution to other auto-immune disease. Autoimm. Rev. 9, 595–599 - PubMed
-
- El-Azhary R. A., Aponte C. C., Nelson A. M., Weaver A. L., Homburger H. A. (2006) Antihistone antibodies in linear scleroderma variants. Int. J. Dermatol. 45, 1296–1299 - PubMed
-
- Neeli I., Khan S. N., Radic M. (2008) Histone deimination as a response to inflammatory stimuli in neutrophils. J. Immunol. 180, 1895–1902 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

