Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents
- PMID: 24671991
- PMCID: PMC3965775
- DOI: 10.1523/JNEUROSCI.5387-13.2014
Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents
Abstract
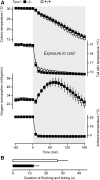
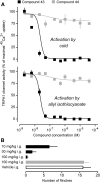
The rodent transient receptor potential ankyrin-1 (TRPA1) channel has been hypothesized to serve as a temperature sensor for thermoregulation in the cold. We tested this hypothesis by using deletion of the Trpa1 gene in mice and pharmacological blockade of the TRPA1 channel in rats. In both Trpa1(-/-) and Trpa1(+/+) mice, severe cold exposure (8°C) resulted in decreases of skin and deep body temperatures to ∼8°C and 13°C, respectively, both temperatures being below the reported 17°C threshold temperature for TRPA1 activation. Under these conditions, Trpa1(-/-) mice had the same dynamics of body temperature as Trpa1(+/+) mice and showed no weakness in the tail skin vasoconstriction response or thermogenic response to cold. In rats, the effects of pharmacological blockade were studied by using two chemically unrelated TRPA1 antagonists: the highly potent and selective compound A967079, which had been characterized earlier, and the relatively new compound 43 ((4R)-1,2,3,4-tetrahydro-4-[3-(3-methoxypropoxy)phenyl]-2-thioxo-5H-indeno[1,2-d]pyrimidin-5-one), which we further characterized in the present study and found to be highly potent (IC50 against cold of ∼8 nm) and selective. Intragastric administration of either antagonist at 30 mg/kg before severe (3°C) cold exposure did not affect the thermoregulatory responses (deep body and tail skin temperatures) of rats, even though plasma concentrations of both antagonists well exceeded their IC50 value at the end of the experiment. In the same experimental setup, blocking the melastatin-8 (TRPM8) channel with AMG2850 (30 mg/kg) attenuated cold-defense mechanisms and led to hypothermia. We conclude that TRPA1 channels do not drive autonomic thermoregulatory responses to cold in rodents.
Keywords: TRPA1; TRPM8; cold exposure; hypothermia; thermoregulation.
Figures

References
-
- Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, Garami A, Bautista D, Gavva NR, Romanovsky AA. Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci. 2012;32:2086–2099. doi: 10.1523/JNEUROSCI.5606-11.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
