A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem
- PMID: 24671996
- PMCID: PMC3965779
- DOI: 10.1523/JNEUROSCI.5071-13.2014
A major external source of cholinergic innervation of the striatum and nucleus accumbens originates in the brainstem
Abstract
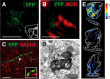
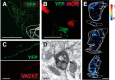
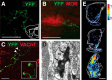
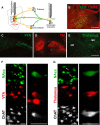
Cholinergic transmission in the striatal complex is critical for the modulation of the activity of local microcircuits and dopamine release. Release of acetylcholine has been considered to originate exclusively from a subtype of striatal interneuron that provides widespread innervation of the striatum. Cholinergic neurons of the pedunculopontine (PPN) and laterodorsal tegmental (LDT) nuclei indirectly influence the activity of the dorsal striatum and nucleus accumbens through their innervation of dopamine and thalamic neurons, which in turn converge at the same striatal levels. Here we show that cholinergic neurons in the brainstem also provide a direct innervation of the striatal complex. By the expression of fluorescent proteins in choline acetyltransferase (ChAT)::Cre(+) transgenic rats, we selectively labeled cholinergic neurons in the rostral PPN, caudal PPN, and LDT. We show that cholinergic neurons topographically innervate wide areas of the striatal complex: rostral PPN preferentially innervates the dorsolateral striatum, and LDT preferentially innervates the medial striatum and nucleus accumbens core in which they principally form asymmetric synapses. Retrograde labeling combined with immunohistochemistry in wild-type rats confirmed the topography and cholinergic nature of the projection. Furthermore, transynaptic gene activation and conventional double retrograde labeling suggest that LDT neurons that innervate the nucleus accumbens also send collaterals to the thalamus and the dopaminergic midbrain, thus providing both direct and indirect projections, to the striatal complex. The differential activity of cholinergic interneurons and cholinergic neurons of the brainstem during reward-related paradigms suggest that the two systems play different but complementary roles in the processing of information in the striatum.
Keywords: cholinergic; innervation; laterodorsal tegmental nucleus; pedunculopontine nucleus; striatum; topography.
Figures



References
-
- Barroso-Chinea P, Rico AJ, Conte-Perales L, Gómez-Bautista V, Luquin N, Sierra S, Roda E, Lanciego JL. Glutamatergic and cholinergic pedunculopontine neurons innervate the thalamic parafascicular nucleus in rats: changes following experimental parkinsonism. Brain Struct Funct. 2011;216:319–330. doi: 10.1007/s00429-011-0317-x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
