Efferent feedback slows cochlear aging
- PMID: 24672005
- PMCID: PMC3965785
- DOI: 10.1523/JNEUROSCI.4923-13.2014
Efferent feedback slows cochlear aging
Abstract
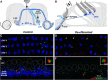
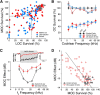
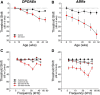
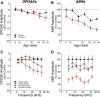
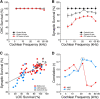
The inner ear receives two types of efferent feedback from the brainstem: one pathway provides gain control on outer hair cells' contribution to cochlear amplification, and the other modulates the excitability of the cochlear nerve. Although efferent feedback can protect hair cells from acoustic injury and thereby minimize noise-induced permanent threshold shifts, most prior studies focused on high-intensity exposures (>100 dB SPL). Here, we show that efferents are essential for long-term maintenance of cochlear function in mice aged 1 year post-de-efferentation without purposeful acoustic overexposure. Cochlear de-efferentation was achieved by surgical lesion of efferent pathways in the brainstem and was assessed by quantitative analysis of immunostained efferent terminals in outer and inner hair cell areas. The resultant loss of efferent feedback accelerated the age-related amplitude reduction in cochlear neural responses, as seen in auditory brainstem responses, and increased the loss of synapses between hair cells and the terminals of cochlear nerve fibers, as seen in confocal analysis of the organ of Corti immunostained for presynaptic and postsynaptic markers. This type of neuropathy, also seen after moderate noise exposure, has been termed "hidden hearing loss", because it does not affect thresholds, but can be seen in the suprathreshold amplitudes of cochlear neural responses, and likely causes problems with hearing in a noisy environment, a classic symptom of age-related hearing loss in humans. Since efferent reflex strength varies among individuals and can be measured noninvasively, a weak reflex may be an important risk factor, and prognostic indicator, for age-related hearing impairment.
Keywords: auditory neuropathy; feedback; hair cells; hearing conservation.
Figures
References
-
- Chays A, Magnan J, Maison S. Absence d'efférences olivocochléaires dans les prélèvements de nerf vestibulaire du patient neurotomisé. JFORL. 2002;51:145–150.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
