Circadian perinatal photoperiod has enduring effects on retinal dopamine and visual function
- PMID: 24672008
- PMCID: PMC3965786
- DOI: 10.1523/JNEUROSCI.4887-13.2014
Circadian perinatal photoperiod has enduring effects on retinal dopamine and visual function
Abstract
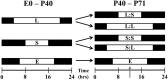
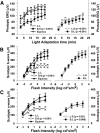
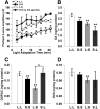
Visual system development depends on neural activity, driven by intrinsic and light-sensitive mechanisms. Here, we examined the effects on retinal function due to exposure to summer- and winter-like circadian light cycles during development and adulthood. Retinal light responses, visual behaviors, dopamine content, retinal morphology, and gene expression were assessed in mice reared in seasonal photoperiods consisting of light/dark cycles of 8:16, 16:8, and 12:12 h, respectively. Mice exposed to short, winter-like, light cycles showed enduring deficits in photopic retinal light responses and visual contrast sensitivity, but only transient changes were observed for scotopic measures. Dopamine levels were significantly lower in short photoperiod mice, and dopaminergic agonist treatment rescued the photopic light response deficits. Tyrosine hydroxylase and Early Growth Response factor-1 mRNA expression were reduced in short photoperiod retinas. Therefore, seasonal light cycles experienced during retinal development and maturation have lasting influence on retinal and visual function, likely through developmental programming of retinal dopamine.
Keywords: circadian; dopamine; electroretinogram; photoperiod; retina; vision.
Figures



References
-
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002;19:593–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
