Cholinergic inputs from Basal forebrain add an excitatory bias to odor coding in the olfactory bulb
- PMID: 24672011
- PMCID: PMC3965788
- DOI: 10.1523/JNEUROSCI.5026-13.2014
Cholinergic inputs from Basal forebrain add an excitatory bias to odor coding in the olfactory bulb
Abstract
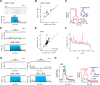
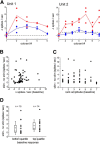
Cholinergic modulation of central circuits is associated with active sensation, attention, and learning, yet the neural circuits and temporal dynamics underlying cholinergic effects on sensory processing remain unclear. Understanding the effects of cholinergic modulation on particular circuits is complicated by the widespread projections of cholinergic neurons to telencephalic structures that themselves are highly interconnected. Here we examined how cholinergic projections from basal forebrain to the olfactory bulb (OB) modulate output from the first stage of sensory processing in the mouse olfactory system. By optogenetically activating their axons directly in the OB, we found that cholinergic projections from basal forebrain regulate OB output by increasing the spike output of presumptive mitral/tufted cells. Cholinergic stimulation increased mitral/tufted cell spiking in the absence of inhalation-driven sensory input and further increased spiking responses to inhalation of odorless air and to odorants. This modulation was rapid and transient, was dependent on local cholinergic signaling in the OB, and differed from modulation by optogenetic activation of cholinergic neurons in basal forebrain, which led to a mixture of mitral/tufted cell excitation and suppression. Finally, bulbar cholinergic enhancement of mitral/tufted cell odorant responses was robust and occurred independent of the strength or even polarity of the odorant-evoked response, indicating that cholinergic modulation adds an excitatory bias to mitral/tufted cells as opposed to increasing response gain or sharpening response spectra. These results are consistent with a role for the basal forebrain cholinergic system in dynamically regulating the sensitivity to or salience of odors during active sensing of the olfactory environment.
Keywords: acetylcholine; mitral cell; modulation; odor coding; optogenetics.
Figures



Similar articles
-
Cell-Type-Specific Modulation of Sensory Responses in Olfactory Bulb Circuits by Serotonergic Projections from the Raphe Nuclei.J Neurosci. 2016 Jun 22;36(25):6820-35. doi: 10.1523/JNEUROSCI.3667-15.2016. J Neurosci. 2016. PMID: 27335411 Free PMC article.
-
Input dependent modulation of olfactory bulb activity by HDB GABAergic projections.Sci Rep. 2020 Jul 1;10(1):10696. doi: 10.1038/s41598-020-67276-z. Sci Rep. 2020. PMID: 32612119 Free PMC article.
-
Inhalation Frequency Controls Reformatting of Mitral/Tufted Cell Odor Representations in the Olfactory Bulb.J Neurosci. 2018 Feb 28;38(9):2189-2206. doi: 10.1523/JNEUROSCI.0714-17.2018. Epub 2018 Jan 26. J Neurosci. 2018. PMID: 29374137 Free PMC article.
-
Odor representation and coding by the mitral/tufted cells in the olfactory bulb.J Zhejiang Univ Sci B. 2024 Oct 15;25(10):824-840. doi: 10.1631/jzus.B2400051. J Zhejiang Univ Sci B. 2024. PMID: 39420520 Free PMC article. Review.
-
The olfactory bulb: coding and processing of odor molecule information.Science. 1999 Oct 22;286(5440):711-5. doi: 10.1126/science.286.5440.711. Science. 1999. PMID: 10531048 Review.
Cited by
-
Cholinergic modulation of spatial learning, memory and navigation.Eur J Neurosci. 2018 Sep;48(5):2199-2230. doi: 10.1111/ejn.14089. Epub 2018 Aug 19. Eur J Neurosci. 2018. PMID: 30055067 Free PMC article. Review.
-
Coherent olfactory bulb gamma oscillations arise from coupling independent columnar oscillators.J Neurophysiol. 2024 Mar 1;131(3):492-508. doi: 10.1152/jn.00361.2023. Epub 2024 Jan 24. J Neurophysiol. 2024. PMID: 38264784 Free PMC article.
-
Cell-Type-Specific Modulation of Sensory Responses in Olfactory Bulb Circuits by Serotonergic Projections from the Raphe Nuclei.J Neurosci. 2016 Jun 22;36(25):6820-35. doi: 10.1523/JNEUROSCI.3667-15.2016. J Neurosci. 2016. PMID: 27335411 Free PMC article.
-
Population imaging at subcellular resolution supports specific and local inhibition by granule cells in the olfactory bulb.Sci Rep. 2016 Jul 8;6:29308. doi: 10.1038/srep29308. Sci Rep. 2016. PMID: 27388949 Free PMC article.
-
NIH Workshop Report: sensory nutrition and disease.Am J Clin Nutr. 2021 Jan 4;113(1):232-245. doi: 10.1093/ajcn/nqaa302. Am J Clin Nutr. 2021. PMID: 33300030 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
