Activation of Kaposi's sarcoma-associated herpesvirus (KSHV) by inhibitors of class III histone deacetylases: identification of sirtuin 1 as a regulator of the KSHV life cycle
- PMID: 24672028
- PMCID: PMC4093851
- DOI: 10.1128/JVI.00219-14
Activation of Kaposi's sarcoma-associated herpesvirus (KSHV) by inhibitors of class III histone deacetylases: identification of sirtuin 1 as a regulator of the KSHV life cycle
Abstract
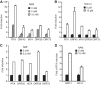
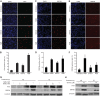
Kaposi's sarcoma-associated herpesvirus (KSHV) establishes persistent latent infection in immunocompetent hosts. Disruption of KSHV latency results in viral lytic replication, which promotes the development of KSHV-related malignancies in immunocompromised individuals. While inhibitors of classes I and II histone deacetylases (HDACs) potently reactivate KSHV from latency, the role of class III HDAC sirtuins (SIRTs) in KSHV latency remains unclear. Here, we examined the effects of inhibitors of SIRTs, nicotinamide (NAM) and sirtinol, on KSHV reactivation from latency. Treatment of latently KSHV-infected cells with NAM or sirtinol induced transcripts and proteins of the master lytic transactivator RTA (ORF50), early lytic genes ORF57 and ORF59, and late lytic gene ORF65 and increased the production of infectious virions. NAM increased the acetylation of histones H3 and H4 as well as the level of the active histone H3 trimethyl Lys4 (H3K4me3) mark but decreased the level of the repressive histone H3 trimethyl Lys27 (H3K27me3) mark in the RTA promoter. Consistent with these results, we detected SIRT1 binding to the RTA promoter. Importantly, knockdown of SIRT1 was sufficient to increase the expression of KSHV lytic genes. Accordingly, the level of the H3K4me3 mark in the RTA promoter was increased following SIRT1 knockdown, while that of the H3K27me3 mark was decreased. Furthermore, SIRT1 interacted with RTA and inhibited RTA transactivation of its own promoter and that of its downstream target, the viral interleukin-6 gene. These results indicate that SIRT1 regulates KSHV latency by inhibiting different stages of viral lytic replication and link the cellular metabolic state with the KSHV life cycle.
Importance: Kaposi's sarcoma-associated herpesvirus (KSHV) is the causal agent of several malignancies, including Kaposi's sarcoma, commonly found in immunocompromised patients. While latent infection is required for the development of KSHV-induced malignancies, viral lytic replication also promotes disease progression. However, the mechanism controlling KSHV latent versus lytic replication remains unclear. In this study, we found that class III histone deacetylases (HDACs), also known as SIRTs, whose activities are linked to the cellular metabolic state, mediate KSHV replication. Inhibitors of SIRTs can reactivate KSHV from latency. SIRTs mediate KSHV latency by epigenetically silencing a key KSHV lytic replication activator, RTA. We found that one of the SIRTs, SIRT1, binds to the RTA promoter to mediate KSHV latency. Knockdown of SIRT1 is sufficient to induce epigenetic remodeling and KSHV lytic replication. SIRT1 also interacts with RTA and inhibits RTA's transactivation function, preventing the expression of its downstream genes. Our results indicate that SIRTs regulate KSHV latency by inhibiting different stages of viral lytic replication and link the cellular metabolic state with the KSHV life cycle.
Figures





References
-
- Cattelan AM, Calabro ML, Gasperini P, Aversa SM, Zanchetta M, Meneghetti F, De Rossi A, Chieco-Bianchi L. 2001. Acquired immunodeficiency syndrome-related Kaposi's sarcoma regression after highly active antiretroviral therapy: biologic correlates of clinical outcome. J. Natl. Cancer Inst. Monogr. 28:44–49 http://jncimono.oxfordjournals.org/content/2000/28/44.long - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

