A complex comprising phosphatidylinositol 4-kinase IIIβ, ACBD3, and Aichi virus proteins enhances phosphatidylinositol 4-phosphate synthesis and is critical for formation of the viral replication complex
- PMID: 24672044
- PMCID: PMC4054359
- DOI: 10.1128/JVI.00208-14
A complex comprising phosphatidylinositol 4-kinase IIIβ, ACBD3, and Aichi virus proteins enhances phosphatidylinositol 4-phosphate synthesis and is critical for formation of the viral replication complex
Abstract
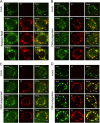
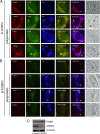
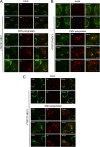
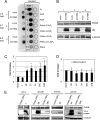
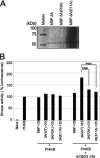
Phosphatidylinositol 4-kinase IIIβ (PI4KB) is a host factor required for the replication of certain picornavirus genomes. We previously showed that nonstructural proteins 2B, 2BC, 2C, 3A, and 3AB of Aichi virus (AiV), a picornavirus, interact with the Golgi protein, acyl-coenzyme A binding domain containing 3 (ACBD3), which interacts with PI4KB. These five viral proteins, ACBD3, PI4KB, and the PI4KB product phosphatidylinositol 4-phosphate (PI4P) colocalize to the AiV RNA replication sites (J. Sasaki et al., EMBO J. 31:754-766, 2012). We here examined the roles of these viral and cellular molecules in the formation of AiV replication complexes. Immunofluorescence microscopy revealed that treatment of AiV polyprotein-expressing cells with a small interfering RNA targeting ACBD3 abolished colocalization of the viral 2B, 2C, and 3A proteins with PI4KB. A PI4KB-specific inhibitor also prevented their colocalization. Virus RNA replication increased the level of cellular PI4P without affecting that of PI4KB, and individual expression of 2B, 2BC, 2C, 3A, or 3AB stimulated PI4P generation. These results suggest that the viral protein/ACBD3/PI4KB complex plays an important role in forming the functional replication complex by enhancing PI4P synthesis. Of the viral proteins, 3A and 3AB were shown to stimulate the in vitro kinase activity of PI4KB through forming a 3A or 3AB/ACBD3/PI4KB complex, whereas the ACBD3-mediated PI4KB activation by 2B and 2C remains to be demonstrated.
Importance: The phosphatidylinositol 4-kinase PI4KB is a host factor required for the replication of certain picornavirus genomes. Aichi virus, a picornavirus belonging to the genus Kobuvirus, forms a complex comprising one of the viral nonstructural proteins 2B, 2BC, 2C, 3A, and 3AB, the Golgi protein ACBD3, and PI4KB to synthesize PI4P at the sites for viral RNA replication. However, the roles of this protein complex in forming the replication complex are unknown. This study showed that virus RNA replication and individual viral proteins enhance the level of cellular PI4P, and suggested that the viral protein/ACBD3/PI4KB complex plays an important role in forming a functional replication complex. Thus, the present study provides a new example of modulation of cellular lipid metabolism by viruses to support the replication of their genomes.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

