Identification of a novel rhabdovirus in Spodoptera frugiperda cell lines
- PMID: 24672045
- PMCID: PMC4054387
- DOI: 10.1128/JVI.00780-14
Identification of a novel rhabdovirus in Spodoptera frugiperda cell lines
Abstract
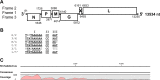
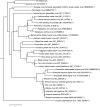
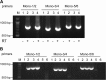
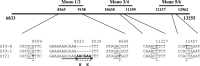
The Sf9 cell line, derived from Spodoptera frugiperda, is used as a cell substrate for biological products, and no viruses have been reported in this cell line after extensive testing. We used degenerate PCR assays and massively parallel sequencing (MPS) to identify a novel RNA virus belonging to the order Mononegavirales in Sf9 cells. Sequence analysis of the assembled virus genome showed the presence of five open reading frames (ORFs) corresponding to the genes for the N, P, M, G, and L proteins in other rhabdoviruses and an unknown ORF of 111 amino acids located between the G- and L-protein genes. BLAST searches indicated that the S. frugiperda rhabdovirus (Sf-rhabdovirus) was related in a limited region of the L-protein gene to Taastrup virus, a newly discovered member of the Mononegavirales from a leafhopper (Hemiptera), and also to plant rhabdoviruses, particularly in the genus Cytorhabdovirus. Phylogenetic analysis of sequences in the L-protein gene indicated that Sf-rhabdovirus is a novel virus that branched with Taastrup virus. Rhabdovirus morphology was confirmed by transmission electron microscopy of filtered supernatant samples from Sf9 cells. Infectivity studies indicated potential transient infection by Sf-rhabdovirus in other insect cell lines, but there was no evidence of entry or virus replication in human cell lines. Sf-rhabdovirus sequences were also found in the Sf21 parental cell line of Sf9 cells but not in other insect cell lines, such as BT1-TN-5B1-4 (Tn5; High Five) cells and Schneider's Drosophila line 2 [D.Mel.(2); SL2] cells, indicating a species-specific infection. The results indicate that conventional methods may be complemented by state-of-the-art technologies with extensive bioinformatics analysis for identification of novel viruses.
Importance: The Spodoptera frugiperda Sf9 cell line is used as a cell substrate for the development and manufacture of biological products. Extensive testing has not previously identified any viruses in this cell line. This paper reports on the identification and characterization of a novel rhabdovirus in Sf9 cells. This was accomplished through the use of next-generation sequencing platforms, de novo assembly tools, and extensive bioinformatics analysis. Rhabdovirus identification was further confirmed by transmission electron microscopy. Infectivity studies showed the lack of replication of Sf-rhabdovirus in human cell lines. The overall study highlights the use of a combinatorial testing approach including conventional methods and new technologies for evaluation of cell lines for unexpected viruses and use of comprehensive bioinformatics strategies for obtaining confident next-generation sequencing results.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- U.S. Food and Drug Administration. 2013. Vaccines, blood & biologics. U.S. Food and Drug Administration, Bethesda, MD: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM0...
-
- U.S. Food and Drug Administration. 2010. Guidance for industry: characterization and qualification of cell substrates and other biological materials used in the production of viral vaccines for infectious disease indications. U.S. Food and Drug Administration, Bethesda, MD: http://www.fda.gov/downloads/biologicsbloodvaccines/guidancecompliancere...
-
- U.S. Food and Drug Administration. 2012. FDA briefing document: Vaccines and Related Biological Products Advisory Committee meeting: cell lines derived from human tumors for vaccine manufacture. U.S. Food and Drug Administration, Bethesda, MD: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMateria...
-
- Schneider I. 1972. Cell lines derived from late embryonic stages of Drosophila melanogaster. J. Embryol. Exp. Morphol. 27:353–365 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

