Coregulatory interactions among CD8α dendritic cells, the latency-associated transcript, and programmed death 1 contribute to higher levels of herpes simplex virus 1 latency
- PMID: 24672046
- PMCID: PMC4054370
- DOI: 10.1128/JVI.00590-14
Coregulatory interactions among CD8α dendritic cells, the latency-associated transcript, and programmed death 1 contribute to higher levels of herpes simplex virus 1 latency
Abstract
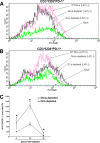
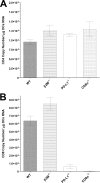
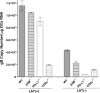
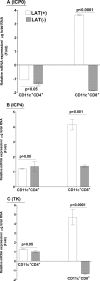
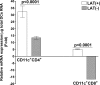
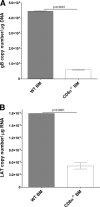
The latency-associated transcript (LAT) of herpes simplex virus 1 (HSV-1), CD8α(+) dendritic cells (DCs), and programmed death 1 (PD-1) have all been implicated in the HSV-1 latency-reactivation cycle. It is not known, however, whether an interaction between LAT and CD8α(+) DCs regulates latency and T-cell exhaustion. To address this question, we used LAT-expressing [LAT(+)] and LAT-negative [LAT(-)] viruses. Depletion of DCs in mice ocularly infected with LAT(+) virus resulted in a reduction in the number of T cells expressing PD-1 in the trigeminal ganglia (TG), whereas depletion of DCs in mice similarly infected with LAT(-) virus did not alter PD-1 expression. CD8α(+) DCs, but not CD4(+) DCs, infected with LAT(+) virus had higher levels of ICP0, ICP4, thymidine kinase (TK), and PD-1 ligand 1 (PD-L1) transcripts than those infected with LAT(-) virus. Coculture of infected bone marrow (BM)-derived DCs from wild-type (WT) mice, but not infected DCs from CD8α(-/-) mice, with WT naive T cells contributed to an increase in PD-1 expression. Transfer of bone marrow from WT mice but not CD8α(-/-) mice to recipient Rag1(-/-) mice increased the number of latent viral genomes in reconstituted mice infected with the LAT(+) virus. Collectively, these data indicated that a reduction in latency correlated with a decline in the levels of CD8α(+) DCs and PD-1 expression. In summary, our results demonstrate an interaction among LAT, PD-1, and CD11c CD8α(+) cells that regulates latency in the TG of HSV-1-infected mice.
Importance: Very little is known regarding the interrelationship of LAT, PD-1, and CD8α(+) DCs and how such interactions might contribute to relative numbers of latent viral genomes. We show here that (i) in both in vivo and in vitro studies, deficiency of CD8α(+) DCs significantly reduced T-cell exhaustion in the presence of LAT(+) virus but not LAT(-) virus; (ii) HSV-1 infectivity was significantly lower in LAT(-)-infected DCs than in their LAT(+)-infected counterparts; and (iii) adoptive transfer of bone marrow (BM) from WT but not CD8α(-/-) mice to recipient Rag1(-/-) mice restored latency to the level in WT mice following infection with LAT(+) virus. These studies point to a key role for CD8α(+) DCs in T-cell exhaustion in the presence of LAT, which leads to larger numbers of latent viral genomes. Thus, altering this negative function of CD8α(+) DCs can potentially be used to generate a more effective vaccine against HSV infection.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

