Optimal immobilization of β-galactosidase onto κ-carrageenan gel beads using response surface methodology and its applications
- PMID: 24672334
- PMCID: PMC3929382
- DOI: 10.1155/2014/571682
Optimal immobilization of β-galactosidase onto κ-carrageenan gel beads using response surface methodology and its applications
Abstract
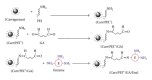
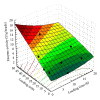
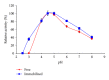
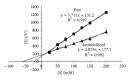
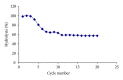
β-Galactosidase (β-gal) was immobilized by covalent binding on novel κ-carrageenan gel beads activated by two-step method; the gel beads were soaked in polyethyleneimine followed by glutaraldehyde. 2(2) full-factorial central composite experiment designs were employed to optimize the conditions for the maximum enzyme loading efficiency. 11.443 U of enzyme/g gel beads was achieved by soaking 40 units of enzyme with the gel beads for eight hours. Immobilization process increased the pH from 4.5 to 5.5 and operational temperature from 50 to 55 °C compared to the free enzyme. The apparent K(m) after immobilization was 61.6 mM compared to 22.9 mM for free enzyme. Maximum velocity Vmax was 131.2 μ mol · min(-1) while it was 177.1 μ mol · min(-1) for free enzyme. The full conversion experiment showed that the immobilized enzyme form is active as that of the free enzyme as both of them reached their maximum 100% relative hydrolysis at 4 h. The reusability test proved the durability of the κ-carrageenan beads loaded with β -galactosidase for 20 cycles with retention of 60% of the immobilized enzyme activity to be more convenient for industrial uses.
Figures



References
-
- Ordoñez JA, Cambero MA, Fernandez L, Garcia ML, Garcia G, Hoz L. Tecnologia de los alimentos (II). Alimentos de Origen Animal. Madrid, Spain: Editorial Sintesis; 1998.
-
- Richmond M, Gray J, Stine C. Beta-galactosidase: review of recent research related to technological application, nutritional concerns, and immobilization. Journal of Dairy Science. 1981;64:1759–1771.
-
- German JH. Milk Powders For the Future. 1997. Applied enzymology of lactose hydrolysis; pp. 81–87.
-
- Sungur S, Akbulut U. Immobilisation of β-galactosidase onto gelatin by glutaraldehyde and chromium(III) acetate. Journal of Chemical Technology and Biotechnology. 1994;59(3):303–306.
-
- Nijipels HH. Lactases and their applications. In: Birch GG, Blakebrough H, Parker KJ, editors. Enzyme and Food Processing. London, UK: Applied Science Publishers; 1981. p. p. 89.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

