Atrial natriuretic factor receptor guanylate cyclase, ANF-RGC, transduces two independent signals, ANF and Ca(2+)
- PMID: 24672425
- PMCID: PMC3955944
- DOI: 10.3389/fnmol.2014.00017
Atrial natriuretic factor receptor guanylate cyclase, ANF-RGC, transduces two independent signals, ANF and Ca(2+)
Abstract
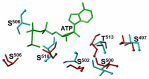
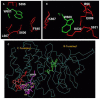
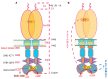
Atrial natriuretic factor receptor guanylate cyclase (ANF-RGC), was the first discovered member of the mammalian membrane guanylate cyclase family. The hallmark feature of the family is that a single protein contains both the site for recognition of the regulatory signal and the ability to transduce it into the production of the second messenger, cyclic GMP. For over two decades, the family has been classified into two subfamilies, the hormone receptor subfamily with ANF-RGC being its paramount member, and the Ca(2+) modulated subfamily, which includes the rod outer segment guanylate cyclases, ROS-GC1 and 2, and the olfactory neuroepithelial guanylate cyclase. ANF-RGC is the receptor and the signal transducer of the most hypotensive hormones, ANF- and B-type natriuretic peptide (BNP). After binding these hormones at the extracellular domain it, at its intracellular domain, signals activation of the C-terminal catalytic module and accelerates the production of cyclic GMP. Cyclic GMP then serves the second messenger role in biological responses of ANF and BNP such as natriuresis, diuresis, vasorelaxation, and anti-proliferation. Very recently another modus operandus for ANF-RGC was revealed. Its crux is that ANF-RGC activity is also regulated by Ca(2+). The Ca(2+) sensor neurocalcin d mediates this signaling mechanism. Strikingly, the Ca(2+) and ANF signaling mechanisms employ separate structural motifs of ANF-RGC in modulating its core catalytic domain in accelerating the production of cyclic GMP. In this review the biochemistry and physiology of these mechanisms with emphasis on cardiovascular regulation will be discussed.
Keywords: atrial natriuretic factor; atrial natriuretic factor receptor guanylate cyclase; calcium; cyclic GMP; neurocalcin δ; signal transduction.
Figures
References
-
- Brenner B. M., Ballermann B. J., Gunning M. E., Zeidel M. L. (1990). Diverse biological actions of atrial natriuretic peptide. Physiol. Rev. 7 665–669 - PubMed
-
- Burnett J. C. Jr., Granger J. P., Opgenorth T. J. (1984). Effect of synthetic atrial natriuretic factor on renal function and rennin release. Am. J. Physiol. 247 F863–F866 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

