Disrupting neuronal transmission: mechanism of DBS?
- PMID: 24672437
- PMCID: PMC3954233
- DOI: 10.3389/fnsys.2014.00033
Disrupting neuronal transmission: mechanism of DBS?
Abstract
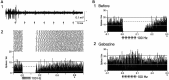
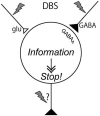
Applying high-frequency stimulation (HFS) to deep brain structure, known as deep brain stimulation (DBS), has now been recognized an effective therapeutic option for a wide range of neurological and psychiatric disorders. DBS targeting the basal ganglia thalamo-cortical loop, especially the internal segment of the globus pallidus (GPi), subthalamic nucleus (STN) and thalamus, has been widely employed as a successful surgical therapy for movement disorders, such as Parkinson's disease, dystonia and tremor. However, the neurophysiological mechanism underling the action of DBS remains unclear and is still under debate: does DBS inhibit or excite local neuronal elements? In this review, we will examine this question and propose the alternative interpretation: DBS dissociates inputs and outputs, resulting in disruption of abnormal signal transmission.
Keywords: basal ganglia; cortico-basal ganglia loop; deep brain stimulation; electrophysiology; globus pallidus; subthalamic nucleus.
Figures



References
-
- Bar-Gad I., Elias S., Vaadia E., Bergman H. (2004). Complex locking rather than complete cessation of neuronal activity in the globus pallidus of a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primate in response to pallidal microstimulation. J. Neurosci. 24, 7410–7419 10.1523/jneurosci.1691-04.2004 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

