Transcriptomics of post-stroke angiogenesis in the aged brain
- PMID: 24672479
- PMCID: PMC3957426
- DOI: 10.3389/fnagi.2014.00044
Transcriptomics of post-stroke angiogenesis in the aged brain
Abstract
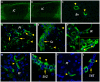
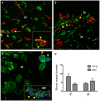
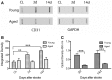
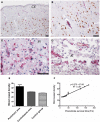
Despite the obvious clinical significance of post-stroke angiogenesis in aged subjects, a detailed transcriptomic analysis of post-stroke angiogenesis has not yet been undertaken in an aged experimental model. In this study, by combining stroke transcriptomics with immunohistochemistry in aged rats and post-stroke patients, we sought to identify an age-specific gene expression pattern that may characterize the angiogenic process after stroke. We found that both young and old infarcted rats initiated vigorous angiogenesis. However, the young rats had a higher vascular density by day 14 post-stroke. "New-for-stroke" genes that were linked to the increased vasculature density in young animals included Angpt2, Angptl2, Angptl4, Cib1, Ccr2, Col4a2, Cxcl1, Lef1, Hhex, Lamc1, Nid2, Pcam1, Plod2, Runx3, Scpep1, S100a4, Tgfbi, and Wnt4, which are required for sprouting angiogenesis, reconstruction of the basal lamina (BL), and the resolution phase. The vast majority of genes involved in sprouting angiogenesis (Angpt2, Angptl4, Cib1, Col8a1, Nrp1, Pcam1, Pttg1ip, Rac2, Runx1, Tnp4, Wnt4); reconstruction of a new BL (Col4a2, Lamc1, Plod2); or tube formation and maturation (Angpt1, Gpc3, Igfbp7, Sparc, Tie2, Tnfsf10), had however, a delayed upregulation in the aged rats. The angiogenic response in aged rats was further diminished by the persistent upregulation of "inflammatory" genes (Cxcl12, Mmp8, Mmp12, Mmp14, Mpeg1, Tnfrsf1a, Tnfrsf1b) and vigorous expression of genes required for the buildup of the fibrotic scar (Cthrc1, Il6ra, Il13ar1, Il18, Mmp2, Rassf4, Tgfb1, Tgfbr2, Timp1). Beyond this barrier, angiogenesis in the aged brains was similar to that in young brains. We also found that the aged human brain is capable of mounting a vigorous angiogenic response after stroke, which most likely reflects the remaining brain plasticity of the aged brain.
Keywords: aging; angiogenesis; stroke; transcriptomics.
Figures


References
-
- Bailey J. R., Bland P. W., Tarlton J. F., Peters I., Moorghen M., Sylvester P. A., et al. (2012). IL-13 promotes collagen accumulation in Crohn’s disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells. PLoS ONE 7:e52332. 10.1371/journal.pone.0052332 - DOI - PMC - PubMed
-
- Basile D. P., Friedrich J. L., Spahic J., Knipe N., Mang H., Leonard E. C., et al. (2011). Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am. J. Physiol. Renal Physiol. 300, 721–733. 10.1152/ajprenal.00546.2010 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

