Identification of carotenoids from the extremely halophilic archaeon Haloarcula japonica
- PMID: 24672517
- PMCID: PMC3956123
- DOI: 10.3389/fmicb.2014.00100
Identification of carotenoids from the extremely halophilic archaeon Haloarcula japonica
Abstract
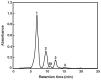
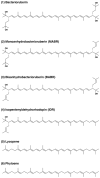
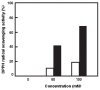
The carotenoids produced by extremely halophilic archaeon Haloarcula japonica were extracted and identified by their chemical, chromatographic, and spectroscopic characteristics (UV-Vis and mass spectrometry). The composition (mol%) was 68.1% bacterioruberin, 22.5% monoanhydrobacterioruberin, 9.3% bisanhydrobacterioruberin, <0.1% isopentenyldehydrorhodopin, and trace amounts of lycopene and phytoene. The in vitro scavenging capacity of a carotenoid, bacterioruberin, extracted from Haloarcula japonica cells against 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals was evaluated. The antioxidant capacity of bacterioruberin was much higher than that of β -carotene.
Keywords: C50 carotenoid; Haloarcula japonica; antioxidant capacity; bacterioruberin; extremely halophilic archaeon.
Figures
References
-
- Burton G. W. (1989). Antioxidant action of carotenoids. J. Nutr. 119 109–111 - PubMed
-
- Das Sarma S., Fleoschmann E. M. (1995). Archaea, a Laboratory Manual. Cold Spring Harbor: Cold Springer Harbor Laboratory Press
LinkOut - more resources
Full Text Sources
Other Literature Sources

