Bioabsorbable stent quo vadis: a case for nano-theranostics
- PMID: 24672583
- PMCID: PMC3966055
- DOI: 10.7150/thno.8137
Bioabsorbable stent quo vadis: a case for nano-theranostics
Abstract
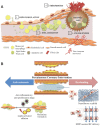
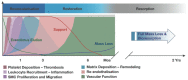
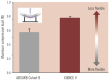
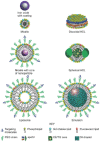
Percutaneous coronary intervention (PCI) is one of the most commonly performed invasive medical procedures in medicine today. Since the first coronary balloon angioplasty in 1977, interventional cardiology has seen a wide array of developments in PCI. Bare metal stents (BMS) were soon superseded by the revolutionary drug-eluting stents (DES), which aimed to address the issue of restenosis found with BMS. However, evidence began to mount against DES, with late-stent thrombosis (ST) rates being higher than that of BMS. The bioabsorbable stent may be a promising alternative, providing vessel patency and support for the necessary time required and thereafter degrade into safe non-toxic compounds which are reabsorbed by the body. This temporary presence provides no triggers for ST, which is brought about by non-endothelialized stent struts and drug polymers remaining in vivo for extended periods of time. Likewise, nano-theranostics incorporated into a bioabsorbable stent of the future may provide an incredibly valuable single platform offering both therapeutic and diagnostic capabilities. Such a stent may allow delivery of therapeutic particles to specific sites thus keeping potential toxicity to a minimum, improved ease of tracking delivery in vivo by embedding imaging agents, controlled rate of therapy release and protection of the implanted therapy. Indeed, nanocarriers may allow an increased therapeutic index as well as offer novel post-stent implantation imaging and diagnostic methods for atherosclerosis, restenosis and thrombosis. It is envisioned that a nano-theranostic stent may well form the cornerstone of future stent designs in clinical practice.
Keywords: bioabsorbable stent; drug-eluting stent; nanotechnology; theranostics.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

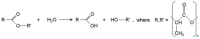




References
-
- Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56:S1–42. - PubMed
-
- Gruntzig A. Transluminal dilatation of coronary-artery stenosis. Lancet. 1978;1:263. - PubMed
-
- Sigwart U, Puel J, Mirkovitch V. et al. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701–706. - PubMed
-
- Serruys PW, Strauss BH, Beatt KJ. et al. Angiographic follow-up after placement of a self-expanding coronary-artery stent. N Engl J Med. 1991;324:13–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

