Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function
- PMID: 24672679
- PMCID: PMC3956959
- DOI: 10.1556/EuJMI.2.2012.2.3
Impact of enzymatic tissue disintegration on the level of surface molecule expression and immune cell function
Abstract
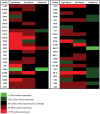
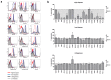
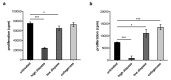
Immunological characterization of immune cells that reside in specific anatomic compartments often requires their isolation from the respective tissue on the basis of enzymatic tissue disintegration. Applying enzymatic digestion of primary splenocytes, we evaluated the impact of collagenase and dispase, two enzymes that are commonly used for the liberation of immune cells from tissues, on the detectability of 48 immunologically relevant surface molecules that are frequently used for flow cytometric identification, isolation, and characterization of immune cell subsets. Whereas collagenase treatment had only minor effects on surface expression of most molecules tested, dispase treatment considerably affected antibody-mediated detectability of the majority of surface markers in subsequent FACS analyses. This effect was long lasting and, in case of high-dose dispase treatment, evident for the majority of surface molecules even after 24 h of in vitro culture. Of note, high-dose dispase treatment not only affected surface expression of certain molecules but also impaired antigen-specific proliferation of CD4(+) and CD8(+) T cells. Together, our data indicate that enzymatic tissue disintegration can have profound effects on the expression of a variety of cell-surface molecules with direct consequences for phenotypic analysis, FACS- and MACS-based target cell isolation, and immune cell function in cell culture experiments.
Keywords: T cell proliferation; collagenase; dispase; enzymatic digestion; flow cytometry; surface molecules.
Figures

References
-
- Kroemer G, Cuende E, Martínez C. Compartmentalization of the peripheral immune system. Adv Immunol. 1993;53:157–216. - PubMed
-
- Bruder D, Westendorf AM, Geffers R, Gruber AD, Gereke M, Enelow RI, Buer J. CD4 T Lymphocyte-mediated lung disease: steady state between pathological and tolerogenic immune reactions. Am J Respir Crit Care Med. 2004 Dec 1;170(11):1145–1152. - PubMed
-
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001 Nov;2(11):1032–1039. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
