The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A₂ via GPR120 receptor to produce prostaglandin E₂ and plays an anti-inflammatory role in macrophages
- PMID: 24673159
- PMCID: PMC4137958
- DOI: 10.1111/imm.12296
The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A₂ via GPR120 receptor to produce prostaglandin E₂ and plays an anti-inflammatory role in macrophages
Abstract
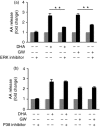
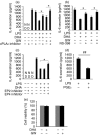
Docosahexaenoic acid (DHA) is one of the major ingredients of fish oil and has been reported to have anti-inflammatory properties mediated through the GPR120 receptor. Whether cytosolic phospholipase A2 (cPLA2 ) and lipid mediators produced from cPLA2 activation are involved in the anti-inflammatory role of DHA in macrophages has not been reported. We report here that DHA and the GPR120 agonist, GW9508, activate cPLA2 and cyclooxygenase 2 (COX-2), and cause prostaglandin E2 (PGE2) release in a murine macrophage cell line RAW264.7 and in human primary monocyte-derived macrophages. DHA and GW9508 activate cPLA2 via GPR120 receptor, G protein Gαq and scaffold protein β-arrestin 2. Extracellular signal-regulated kinase 1/2 activation is involved in DHA- and GW9508-induced cPLA2 activation, but not p38 mitogen-activated protein kinase. The anti-inflammatory role of DHA and GW9508 is in part via activation of cPLA2 , COX-2 and production of PGE2 as a cPLA2 inhibitor or a COX-2 inhibitor partially reverses the DHA- and GW9508-induced inhibition of lipopolysaccharide-induced interleukin-6 secretion. The cPLA2 product arachidonic acid and PGE2 also play an anti-inflammatory role. This effect of PGE2 is partially through inhibition of the nuclear factor-κB signalling pathway and through the EP4 receptor of PGE2 because an EP4 inhibitor or knock-down of EP4 partially reverses DHA inhibition of lipopolysaccharide-induced interleukin-6 secretion. Hence, DHA has an anti-inflammatory effect partially through induction of PGE2.
Keywords: cytokines; inflammation; lipid mediators; signal transduction.
Published 2014. This article is a U.S. Government work and is in the public domain in the USA.
Figures







References
-
- Fritsche K. Fatty acids as modulators of the immune response. Annu Rev Nutr. 2006;26:45–73. - PubMed
-
- Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32:203–27. - PubMed
-
- Calder PC. Long-chain fatty acids and inflammation. Proc Nutr Soc. 2012;71:284–9. - PubMed
-
- Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141:272–82. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

