Comparison of peptide-major histocompatibility complex tetramers and dextramers for the identification of antigen-specific T cells
- PMID: 24673376
- PMCID: PMC4089154
- DOI: 10.1111/cei.12339
Comparison of peptide-major histocompatibility complex tetramers and dextramers for the identification of antigen-specific T cells
Abstract
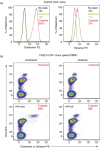
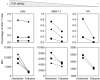
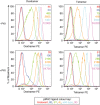
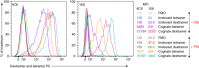
Fluorochrome-conjugated peptide-major histocompatibility complex (pMHC) multimers are widely used for flow cytometric visualization of antigen-specific T cells. The most common multimers, streptavidin-biotin-based 'tetramers', can be manufactured readily in the laboratory. Unfortunately, there are large differences between the threshold of T cell receptor (TCR) affinity required to capture pMHC tetramers from solution and that which is required for T cell activation. This disparity means that tetramers sometimes fail to stain antigen-specific T cells within a sample, an issue that is particularly problematic when staining tumour-specific, autoimmune or MHC class II-restricted T cells, which often display TCRs of low affinity for pMHC. Here, we compared optimized staining with tetramers and dextramers (dextran-based multimers), with the latter carrying greater numbers of both pMHC and fluorochrome per molecule. Most notably, we find that: (i) dextramers stain more brightly than tetramers; (ii) dextramers outperform tetramers when TCR-pMHC affinity is low; (iii) dextramers outperform tetramers with pMHC class II reagents where there is an absence of co-receptor stabilization; and (iv) dextramer sensitivity is enhanced further by specific protein kinase inhibition. Dextramers are compatible with current state-of-the-art flow cytometry platforms and will probably find particular utility in the fields of autoimmunity and cancer immunology.
Keywords: T cell receptors; T cells; autoimmunity; diabetes; tumour immunology.
© 2014 The Authors. Clinical & Experimental Immunology published by John Wiley & Sons Ltd on behalf of British Society for Immunology.
Figures




References
-
- Klenerman P, Cerundolo V, Dunbar PR. Tracking T cells with tetramers: new tales from new tools. Nat Rev Immunol. 2002;2:263–272. - PubMed
-
- Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. - PubMed
-
- Batard P, Peterson DA, Devevre E, et al. Dextramers: new generation of fluorescent MHC class I/peptide multimers for visualization of antigen-specific CD8+ T cells. J Immunol Methods. 2006;310:136–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

