Loading of nuclear autoantigens prototypically recognized by systemic lupus erythematosus sera into late apoptotic vesicles requires intact microtubules and myosin light chain kinase activity
- PMID: 24673456
- PMCID: PMC4260895
- DOI: 10.1111/cei.12342
Loading of nuclear autoantigens prototypically recognized by systemic lupus erythematosus sera into late apoptotic vesicles requires intact microtubules and myosin light chain kinase activity
Abstract
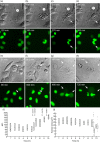
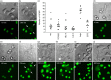
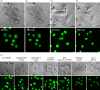
Most cases of systemic lupus erythematosus (SLE) are characterized by an impaired clearance of apoptotic cells in various tissues. Non-cleared apoptotic waste is considered an immunogen driving the autoimmune response in patients with SLE. During the execution of apoptosis, membrane blebs are formed and filled with cellular components. Here, we evaluate the cytoskeletal pathway(s) responsible for the loading of SLE prototypic nuclear autoantigens into the apoptotic cell-derived membranous vesicles (ACMV) generated during late phases of apoptosis. HeLa cells expressing a fusion protein of histone H2B with green fluorescent protein (GFP) were irradiated with ultraviolet (UV)-B to induce apoptosis. The appearance and trafficking of chromatin-derived material was monitored by fluorescence microscopy. Specific inhibitors of cytoskeletal pathways were employed to identify the motile elements involved in translocation and trafficking of the nuclear components. We observed that immediately after their appearance the ACMV did not contain histone H2B(GFP) ; in this phase the fluorescence was contained in the nuclear remnants and the cytoplasm. Within consecutive minutes the ACMV were loaded with chromatin-derived material, whereas the loading of simultaneously created ACMV with histone H2B(GFP) was not uniform. Some ACMV were preferentially filled and, consequently, showed a remarkably higher histone H2B(GFP) accumulation. Inhibitors of the cytoskeleton revealed that functional microtubules and myosin light chain kinase are required for nuclear shrinkage and loading of nuclear material into the ACMV, respectively.
Keywords: apoptosis; blebbing; nuclear autoantigens; systemic lupus erythematosus.
© 2014 British Society for Immunology.
Figures


References
-
- Wayaku T, Hasegawa M, Kaji K, et al. Antigen specificity of antihistone antibodies in connective tissue disease patients with anti-U1RNP antibodies. Rheumatol Int. 2007;28:113–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

