Efficacy and safety of memantine in patients with moderate-to-severe Alzheimer's disease: results of a pooled analysis of two randomized, double-blind, placebo-controlled trials in Japan
- PMID: 24673497
- PMCID: PMC4025599
- DOI: 10.1517/14656566.2014.902446
Efficacy and safety of memantine in patients with moderate-to-severe Alzheimer's disease: results of a pooled analysis of two randomized, double-blind, placebo-controlled trials in Japan
Abstract
Background: With the increase in the aging population, there is a pressing need to provide effective treatment options for individuals with Alzheimer's disease (AD). Memantine is an N-methyl-D-aspartate receptor antagonist used to treat AD in > 80 countries worldwide, and studies in the USA and Europe have shown it to be effective in improving language deficits; however, there are currently no data on language improvements in Japanese patients treated with memantine.
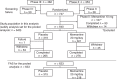
Objectives: To clarify the efficacy and safety of memantine in Japanese outpatients with moderate to severe AD, using a pooled analysis of two multicenter randomized placebo-controlled trials, a phase 2 dose-finding study and a phase 3 study.
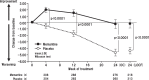
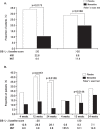
Results: The final analysis comprised 633 patients (318 receiving memantine and 315 placebo). Memantine produced better outcomes in terms of Severe Impairment Battery-Japanese version, Clinician's Interview-Based Impression of Change plus-Japanese version, Behavioral Pathology in AD Rating Scale, and language scores, versus placebo. The overall incidence of adverse events and adverse reactions was similar between groups.
Conclusion: In this pooled analysis of Japanese patients, memantine achieved better outcomes than placebo in terms of cognition, including attention, praxis, visuospatial ability and language, and behavioral and psychological symptoms, including activity disturbances and aggressiveness.
Figures






References
-
- Reisberg B, Doody R, Stöffler A, for the Memantine Study Group Memantine in moderate-to-severe Alzheimer's disease. N. Engl J Med. 2003;348:1333–41. - PubMed
-
- Tariot PN, Farlow MR, Grossberg GT, for the Memantine Study Group Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317–24. - PubMed
-
- Winblad B, Jones RW, Wirth Y. Memantine in moderate to severe Alzheimer's disease: a meta-analysis of randomised clinical trials. Dement Geriatr Cogn Disord. 2007;24:20–7. - PubMed
-
- Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system - too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. - PubMed
-
- Kitamura S, Homma A, Nakamura Y. Phase II study of memantine hydrochloride, a new NMDA receptor antagonist, in patients with moderate to severe Alzheimer's disease –Efficacy, safety and recommended dose. Jpn J Geriatr Psychiat. 2011;22:453–63.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
