Laser-engineered dissolving microneedle arrays for protein delivery: potential for enhanced intradermal vaccination
- PMID: 24673568
- PMCID: PMC7938851
- DOI: 10.1111/jphp.12248
Laser-engineered dissolving microneedle arrays for protein delivery: potential for enhanced intradermal vaccination
Abstract
Objectives: We aimed to highlight the utility of novel dissolving microneedle (MN)-based delivery systems for enhanced transdermal protein delivery. Vaccination remains the most accepted and effective approach in offering protection from infectious diseases. In recent years, much interest has focused on the possibility of using minimally invasive MN technologies to replace conventional hypodermic vaccine injections.
Methods: The focus of this study was exploitation of dissolving MN array devices fabricated from 20% w/w poly(methyl vinyl ether/maleic acid) using a micromoulding technique, for the facilitated delivery of a model antigen, ovalbumin (OVA).
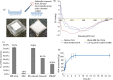
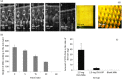
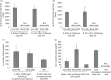
Key findings: A series of in-vitro and in-vivo experiments were designed to demonstrate that MN arrays loaded with OVA penetrated the stratum corneum and delivered their payload systemically. The latter was evidenced by the activation of both humoral and cellular inflammatory responses in mice, indicated by the production of immunoglobulins (IgG, IgG1, IgG2a) and inflammatory cytokines, specifically interferon-gamma and interleukin-4. Importantly, the structural integrity of the OVA following incorporation into the MN arrays was maintained.
Conclusion: While enhanced manufacturing strategies are required to improve delivery efficiency and reduce waste, dissolving MN are a promising candidate for 'reduced-risk' vaccination and protein delivery strategies.
Keywords: laser-engineering; microneedles; protein delivery; vaccination.
© 2014 Royal Pharmaceutical Society.
Figures

References
-
- Donnelly RF, et al. Microneedle-Mediated Transdermal and Intradermal Drug Delivery. New Jersey: John Wiley & Sons, 2012.
-
- Ito Y, et al. Transdermal insulin application system with dissolving microneedles. Diabetes Technol Ther 2012; 14: 891–899. - PubMed
-
- Liu S, et al. The development and characteristics of novel microneedle arrays fabricated from hyaluronic acid, and their application in the transdermal delivery of insulin. J Control Release 2012; 161: 933–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/K020234/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT094085MA/WT_/Wellcome Trust/United Kingdom
- BB/E020534/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/FOF/287/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

