Melanoma cell lysate induces CCR7 expression and in vivo migration to draining lymph nodes of therapeutic human dendritic cells
- PMID: 24673602
- PMCID: PMC4080955
- DOI: 10.1111/imm.12264
Melanoma cell lysate induces CCR7 expression and in vivo migration to draining lymph nodes of therapeutic human dendritic cells
Abstract
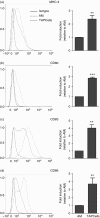
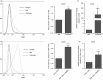
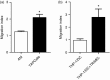
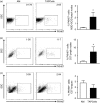
We have previously reported a novel method for the production of tumour-antigen-presenting cells (referred to as TAPCells) that are currently being used in cancer therapy, using an allogeneic melanoma-derived cell lysate (referred to as TRIMEL) as an antigen provider and activation factor. It was recently demonstrated that TAPCell-based immunotherapy induces T-cell-mediated immune responses resulting in improved long-term survival of stage IV melanoma patients. Clinically, dendritic cell (DC) migration from injected sites to lymph nodes is an important requirement for an effective anti-tumour immunization. This mobilization of DCs is mainly driven by the C-C chemokine receptor type 7 (CCR7), which is up-regulated on mature DCs. Using flow cytometry and immunohistochemistry, we investigated if TRIMEL was capable of inducing the expression of the CCR7 on TAPCells and enhancing their migration in vitro, as well as their in vivo relocation to lymph nodes in an ectopic xenograft animal model. Our results confirmed that TRIMEL induces a phenotypic maturation and increases the expression of surface CCR7 on melanoma patient-derived DCs, and also on the monocytic/macrophage cell line THP-1. Moreover, in vitro assays showed that TRIMEL-stimulated DCs and THP-1 cells were capable of migrating specifically in the presence of the CCR7 ligand CCL19. Finally, we demonstrated that TAPCells could migrate in vivo from the injection site into the draining lymph nodes. This work contributes to an increased understanding of the biology of DCs produced ex vivo allowing the design of new strategies for effective DC-based vaccines for treating aggressive melanomas.
Keywords: CCR7; dendritic cells; immunotherapy; melanoma; migration; tumour lysates.
© 2014 John Wiley & Sons Ltd.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

