Overexpression of DNA ligase III in mitochondria protects cells against oxidative stress and improves mitochondrial DNA base excision repair
- PMID: 24674627
- PMCID: PMC5156482
- DOI: 10.1016/j.dnarep.2014.01.015
Overexpression of DNA ligase III in mitochondria protects cells against oxidative stress and improves mitochondrial DNA base excision repair
Abstract
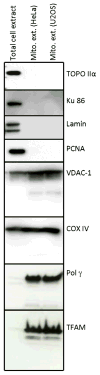
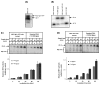
Base excision repair (BER) is the most prominent DNA repair pathway in human mitochondria. BER also results in a temporary generation of AP-sites, single-strand breaks and nucleotide gaps. Thus, incomplete BER can result in the generation of DNA repair intermediates that can disrupt mitochondrial DNA replication and transcription and generate mutations. We carried out BER analysis in highly purified mitochondrial extracts from human cell lines U2OS and HeLa, and mouse brain using a circular DNA substrate containing a lesion at a specific position. We found that DNA ligation is significantly slower than the preceding mitochondrial BER steps. Overexpression of DNA ligase III in mitochondria improved the rate of overall BER, increased cell survival after menadione induced oxidative stress and reduced autophagy following the inhibition of the mitochondrial electron transport chain complex I by rotenone. Our results suggest that the amount of DNA ligase III in mitochondria may be critical for cell survival following prolonged oxidative stress, and demonstrate a functional link between mitochondrial DNA damage and repair, cell survival upon oxidative stress, and removal of dysfunctional mitochondria by autophagy.
Keywords: Autophagy; Cell survival; Mitochondrial DNA repair intermediates; Oxidative stress.
Copyright © 2014. Published by Elsevier B.V.
Conflict of interest statement
statement The authors declare no conflict of interest.
Figures




References
-
- McFarland R, Taylor RW, Turnbull DM. Mitochondrial disease – its impact, etiology, and pathology. Curr Top Dev Biol. 2007;77:113–155. - PubMed
-
- Holt IJ, Harding AE, Morgan-Hughes JA. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988;331:717–719. - PubMed
-
- Zeviani M, Moraes CT, DiMauro S, Nakase H, Bonilla E, Schon EA, Rowland LP. Deletions of mitochondrial DNA in Kearns–Sayre syndrome. Neurology. 1988;38:1339–1346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

