An open-label phase II study evaluating the safety and efficacy of ramucirumab combined with mFOLFOX-6 as first-line therapy for metastatic colorectal cancer
- PMID: 24674871
- PMCID: PMC3983832
- DOI: 10.1634/theoncologist.2014-0028
An open-label phase II study evaluating the safety and efficacy of ramucirumab combined with mFOLFOX-6 as first-line therapy for metastatic colorectal cancer
Abstract
Background: Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR-2) are believed to mediate angiogenesis in colorectal cancer (CRC). Ramucirumab (RAM; IMC-1121B) is a human IgG1 monoclonal antibody that inhibits VEGF ligand binding to VEGFR-2, inhibiting VEGFR-2 activation and signaling.
Methods: Patients with metastatic CRC, Eastern Cooperative Oncology Group performance status 0-1, and adequate organ function who had not received chemotherapy for metastatic disease received RAM and the modified FOLFOX-6 regimen every 2 weeks. Endpoints included progression-free survival (PFS), objective response rate, overall survival, and safety. The sample size was based on a potentially improved median PFS from 8 months to 11 months.
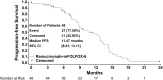
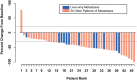
Results: Forty-eight patients received therapy. Median PFS was 11.5 months (95% confidence interval [CI]: 8.6-13.1 months). The objective response rate was 58.3% (95% CI: 43.21-72.39). The disease control rate (complete or partial response plus stable disease) was 93.8% (95% CI: 82.8-98.7). Median overall survival was 20.4 months (95% CI: 18.5-25.1 months). The most frequent grade 3-4 adverse events included neutropenia (grade 3: 33.3%; grade 4: 8.3%), hypertension (grade 3: 16.7%), and neuropathy (grade 3: 12.5%). Two patients died during the study due to myocardial infarction and cardiopulmonary arrest.
Conclusion: RAM may enhance the efficacy of modified FOLFOX-6 chemotherapy with an acceptable safety profile in metastatic CRC.
Figures
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

