Immunological memory within the innate immune system
- PMID: 24674969
- PMCID: PMC4194120
- DOI: 10.1002/embj.201387651
Immunological memory within the innate immune system
Abstract
Immune memory has traditionally been the domain of the adaptive immune system, present only in antigen-specific T and B cells. The purpose of this review is to summarize the evidence for immunological memory in lower organisms (which are not thought to possess adaptive immunity) and within specific cell subsets of the innate immune system. A special focus will be given to recent findings in both mouse and humans for specificity and memory in natural killer (NK) cells, which have resided under the umbrella of innate immunity for decades. The surprising longevity and enhanced responses of previously primed NK cells will be discussed in the context of several immunization settings.
Keywords: NK cells; immunity; innate immunity; memory.
© 2014 The Authors.
Figures


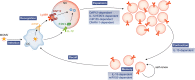
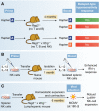
References
-
- Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology. 7th edn. Philadelphia, PA: Saunders/Elsevier; 2012.
-
- Abbas AK, Lichtman AH, Pillai S. Basic Immunology: Functions and Disorders of the Immune System. 4th edn. Philadelphia, PA: Elsevier/Saunders; 2014.
-
- Ahmed R, Akondy RS. Insights into human CD8(+) T-cell memory using the yellow fever and smallpox vaccines. Immunol Cell Biol. 2011;89:340–345. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

