The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer
- PMID: 24675041
- PMCID: PMC4068971
- DOI: 10.1158/2159-8290.CD-13-0846
The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer
Abstract
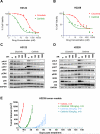
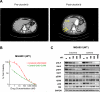
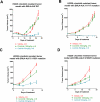
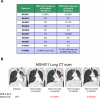
Non-small cell lung cancers (NSCLC) harboring anaplastic lymphoma kinase (ALK) gene rearrangements invariably develop resistance to the ALK tyrosine kinase inhibitor (TKI) crizotinib. Herein, we report the first preclinical evaluation of the next-generation ALK TKI, ceritinib (LDK378), in the setting of crizotinib resistance. An interrogation of in vitro and in vivo models of acquired resistance to crizotinib, including cell lines established from biopsies of patients with crizotinib-resistant NSCLC, revealed that ceritinib potently overcomes crizotinib-resistant mutations. In particular, ceritinib effectively inhibits ALK harboring L1196M, G1269A, I1171T, and S1206Y mutations, and a cocrystal structure of ceritinib bound to ALK provides structural bases for this increased potency. However, we observed that ceritinib did not overcome two crizotinib-resistant ALK mutations, G1202R and F1174C, and one of these mutations was identified in 5 of 11 biopsies from patients with acquired resistance to ceritinib. Altogether, our results demonstrate that ceritinib can overcome crizotinib resistance, consistent with clinical data showing marked efficacy of ceritinib in patients with crizotinib-resistant disease.
Significance: The second-generation ALK inhibitor ceritinib can overcome several crizotinib-resistant mutations and is potent against several in vitro and in vivo laboratory models of acquired resistance to crizotinib. These findings provide the molecular basis for the marked clinical activity of ceritinib in patients with ALK-positive NSCLC with crizotinib-resistant disease. Cancer Discov; 4(6); 662-73. ©2014 AACR. See related commentary by Ramalingam and Khuri, p. 634 This article is highlighted in the In This Issue feature, p. 621.
©2014 American Association for Cancer Research.
Figures


References
-
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

