Failure to induce apoptosis via BCL-2 family proteins underlies lack of efficacy of combined MEK and PI3K inhibitors for KRAS-mutant lung cancers
- PMID: 24675361
- PMCID: PMC4046322
- DOI: 10.1158/0008-5472.CAN-13-3728
Failure to induce apoptosis via BCL-2 family proteins underlies lack of efficacy of combined MEK and PI3K inhibitors for KRAS-mutant lung cancers
Abstract
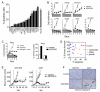
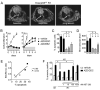
Although several groups have demonstrated that concomitant use of MEK and phosphoinositide 3-kinase (PI3K) inhibitors (MEKi/PI3Ki) can induce dramatic tumor regressions in mouse models of KRAS-mutant non-small cell lung cancer (NSCLC), ongoing clinical trials investigating this strategy have been underwhelming to date. While efficacy may be hampered by a narrow therapeutic index, the contribution of biologic heterogeneity in the response of KRAS-mutant NSCLCs to MEKi/PI3Ki has been largely unexplored. In this study, we find that most human KRAS-mutant NSCLC cell lines fail to undergo marked apoptosis in response to MEKi/PI3Ki, which is key for tumor responsiveness in vivo. This heterogeneity of apoptotic response occurs despite relatively uniform induction of growth arrest. Using a targeted short hairpin RNA screen of BCL-2 family members, we identify BIM, PUMA, and BCL-XL as key regulators of the apoptotic response induced by MEKi/PI3Ki, with decreased expression of BIM and PUMA relative to BCL-XL in cell lines with intrinsic resistance. In addition, by modeling adaptive resistance to MEKi/PI3Ki both in vitro and in vivo, we find that, upon the development of resistance, tumors have a diminished apoptotic response due to downregulation of BIM and PUMA. These results suggest that the inability to induce apoptosis may limit the effectiveness of MEKi/PI3Ki for KRAS-mutant NSCLCs by contributing to intrinsic and adaptive resistance to this therapy.
©2014 American Association for Cancer Research.
Figures
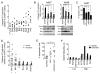


References
-
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–8. - PubMed
-
- Young A, Lyons J, Miller AL, Phan VT, Alarcon IR, McCormick F. Ras signaling and therapies. Advances in cancer research. 2009;102:1–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC2 CA147940/CA/NCI NIH HHS/United States
- R01CA137181/CA/NCI NIH HHS/United States
- P50CA090578/CA/NCI NIH HHS/United States
- R01 CA122794/CA/NCI NIH HHS/United States
- R01 CA137181/CA/NCI NIH HHS/United States
- R01CA122794/CA/NCI NIH HHS/United States
- 1U01CA141457-01/CA/NCI NIH HHS/United States
- R01 CA166480/CA/NCI NIH HHS/United States
- R01 CA140594/CA/NCI NIH HHS/United States
- P50 CA090578/CA/NCI NIH HHS/United States
- R01CA137008-01/CA/NCI NIH HHS/United States
- 1RC2CA147940/CA/NCI NIH HHS/United States
- R01CA140594/CA/NCI NIH HHS/United States
- R01 CA137008/CA/NCI NIH HHS/United States
- U01 CA141457/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

