Comprehensive analyses of ventricular myocyte models identify targets exhibiting favorable rate dependence
- PMID: 24675446
- PMCID: PMC3967944
- DOI: 10.1371/journal.pcbi.1003543
Comprehensive analyses of ventricular myocyte models identify targets exhibiting favorable rate dependence
Abstract
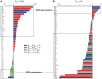
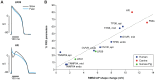
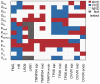
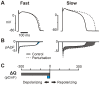
Reverse rate dependence is a problematic property of antiarrhythmic drugs that prolong the cardiac action potential (AP). The prolongation caused by reverse rate dependent agents is greater at slow heart rates, resulting in both reduced arrhythmia suppression at fast rates and increased arrhythmia risk at slow rates. The opposite property, forward rate dependence, would theoretically overcome these parallel problems, yet forward rate dependent (FRD) antiarrhythmics remain elusive. Moreover, there is evidence that reverse rate dependence is an intrinsic property of perturbations to the AP. We have addressed the possibility of forward rate dependence by performing a comprehensive analysis of 13 ventricular myocyte models. By simulating populations of myocytes with varying properties and analyzing population results statistically, we simultaneously predicted the rate-dependent effects of changes in multiple model parameters. An average of 40 parameters were tested in each model, and effects on AP duration were assessed at slow (0.2 Hz) and fast (2 Hz) rates. The analysis identified a variety of FRD ionic current perturbations and generated specific predictions regarding their mechanisms. For instance, an increase in L-type calcium current is FRD when this is accompanied by indirect, rate-dependent changes in slow delayed rectifier potassium current. A comparison of predictions across models identified inward rectifier potassium current and the sodium-potassium pump as the two targets most likely to produce FRD AP prolongation. Finally, a statistical analysis of results from the 13 models demonstrated that models displaying minimal rate-dependent changes in AP shape have little capacity for FRD perturbations, whereas models with large shape changes have considerable FRD potential. This can explain differences between species and between ventricular cell types. Overall, this study provides new insights, both specific and general, into the determinants of AP duration rate dependence, and illustrates a strategy for the design of potentially beneficial antiarrhythmic drugs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Waldo AL, Camm AJ, deRuyter H, Friedman PL, MacNeil DJ, Pauls JF, Pitt B, Pratt CM, Schwartz PJ, Veltri EP (1996) Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet 348: 7–12. - PubMed
-
- Guillaume P, Goineau S, Froget G (2013) An overview of QT interval assessment in safety pharmacology. Curr Protoc Pharmacol Chapter 10: Unit 10 17. - PubMed
-
- Hondeghem LM (2007) Relative contributions of TRIaD and QT to proarrhythmia. J Cardiovasc Electrophysiol 18: 655–657. - PubMed
-
- Hondeghem LM, Snyders DJ (1990) Class III antiarrhythmic agents have a lot of potential but a long way to go. Reduced effectiveness and dangers of reverse use dependence. Circulation 81: 686–690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

