p53 Induces skin aging by depleting Blimp1+ sebaceous gland cells
- PMID: 24675459
- PMCID: PMC3973209
- DOI: 10.1038/cddis.2014.87
p53 Induces skin aging by depleting Blimp1+ sebaceous gland cells
Abstract
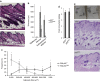
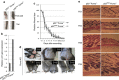
p53 is an important inducer of organismal aging. However, its roles in the aging of skin remain unclear. Here we show that mice with chronic activation of p53 develop an aging phenotype in the skin associated with a reduction of subcutaneous fat and loss of sebaceous gland (SG). The reduction in the fat layer may result from the decrease of mammalian TOR complex 1 (mTORC1) activity accompanied by elevated expression of energy expenditure genes, and possibly as compensatory effects, leading to the elevation of peroxisome proliferator-activated receptor (PPAR)γ, an inducer of sebocyte differentiation. In addition, Blimp1(+) sebocytes become depleted concomitantly with an increase in cellular senescence, which can be reversed by PPARγ antagonist (BADGE) treatment. Therefore, our results indicate that p53-mediated aging of the skin involves not only thinning through the loss of subdermal fat, but also xerosis or drying of the skin through declining sebaceous gland activity.
Figures




References
-
- Baumann L. Skin ageing and its treatment. J Pathol. 2007;211:241–251. - PubMed
-
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, et al. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. - PubMed
-
- Jahoda CA, Christiano AM. Niche crosstalk: intercellular signals at the hair follicle. Cell. 2011;146:678–681. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

