Doxorubicin enhances Snail/LSD1-mediated PTEN suppression in a PARP1-dependent manner
- PMID: 24675890
- PMCID: PMC4111717
- DOI: 10.4161/cc.28619
Doxorubicin enhances Snail/LSD1-mediated PTEN suppression in a PARP1-dependent manner
Abstract
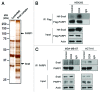
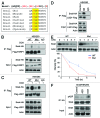
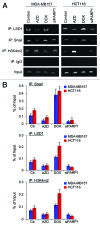
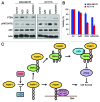
The transcription factor Snail not only functions as a master regulator of epithelial-mesenchymal transition (EMT), but also mediates cell proliferation and survival. While previous studies have showed that Snail protects tumor cells from apoptosis through transcriptional repression of PTEN, the specific mechanism remains unclear. In this study, we demonstrated that Snail cooperates with LSD1 to repress PTEN in a PARP1-dependent manner. Upon doxorubicin treatment, Snail becomes tightly associated with PARP1 through its pADPr-binding motif and is subject to poly(ADP-ribosyl)ation. This modification can enhance Snail-LSD1 interaction and promote the recruitment of LSD1 to PTEN promoter, where LSD1 removes methylation on histone H3 lysine 4 for transcription repression. Furthermore, treatment of tumor cells with PARP1 inhibitor AZD2281 can compromise doxorubicin-induced PTEN suppression and enhance the inhibitory effect of doxorubicin. Together, we proposed a tentative drug-resistant mechanism through which tumor cells defend themselves against DNA damage-induced apoptosis. PARP1 inhibitors in combination with DNA damaging reagents might represent a promising treatment strategy targeting tumors with over-activated Snail and LSD1.
Keywords: LSD1; PARP1; PTEN; Snail; poly(ADP-ribosyl)ation.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
