Correction of distortion in flattened representations of the cortical surface allows prediction of V1-V3 functional organization from anatomy
- PMID: 24676149
- PMCID: PMC3967932
- DOI: 10.1371/journal.pcbi.1003538
Correction of distortion in flattened representations of the cortical surface allows prediction of V1-V3 functional organization from anatomy
Abstract
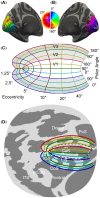
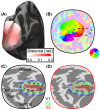
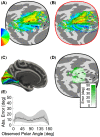
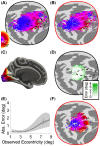
Several domains of neuroscience offer map-like models that link location on the cortical surface to properties of sensory representation. Within cortical visual areas V1, V2, and V3, algebraic transformations can relate position in the visual field to the retinotopic representation on the flattened cortical sheet. A limit to the practical application of this structure-function model is that the cortex, while topologically a two-dimensional surface, is curved. Flattening of the curved surface to a plane unavoidably introduces local geometric distortions that are not accounted for in idealized models. Here, we show that this limitation is overcome by correcting the geometric distortion induced by cortical flattening. We use a mass-spring-damper simulation to create a registration between functional MRI retinotopic mapping data of visual areas V1, V2, and V3 and an algebraic model of retinotopy. This registration is then applied to the flattened cortical surface anatomy to create an anatomical template that is linked to the algebraic retinotopic model. This registered cortical template can be used to accurately predict the location and retinotopic organization of these early visual areas from cortical anatomy alone. Moreover, we show that prediction accuracy remains when extrapolating beyond the range of data used to inform the model, indicating that the registration reflects the retinotopic organization of visual cortex. We provide code for the mass-spring-damper technique, which has general utility for the registration of cortical structure and function beyond the visual cortex.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Wandell BA, Dumoulin SO, Brewer AA (2007) Visual Field Maps in Human Cortex. Neuron 56: 366–383. - PubMed
-
- Dougherty RF, Koch VM, Brewer AA, Fischer B, Modersitzki J, et al. (2003) Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J Vis 3: 1–1. - PubMed
-
- Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9: 179–194. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

