Development of a proficiency testing program for the HIV-1 BED incidence assay in China
- PMID: 24676229
- PMCID: PMC3968539
- DOI: 10.1038/srep04512
Development of a proficiency testing program for the HIV-1 BED incidence assay in China
Abstract
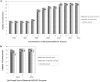
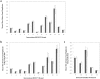
The HIV-1 BED incidence assay was adopted in China in 2005 for HIV-1 incidence surveillance. A proficiency testing (PT) program was established in 2006 to provide quality assurance services. The BED PT program consisted of two components, an international program provided by the U.S. Centers for Disease Control and Prevention from 2006 and a domestic program started by the National HIV/HCV Reference Laboratory in 2011. Each PT panel consisted of eight coded specimens distributed to participating laboratories semi-annually, and testing results were collected and analyzed. The number of participating laboratories increased progressively from 2006 to 2012. The Chinese HIV-1 incidence laboratory network performed satisfactorily both in international and domestic PT programs. We also demonstrated that the BED assay was highly reproducible among participating laboratories. Our success and lessons learned can be readily replicated in other countries or regions contemplating the establishment of a PT program for assay-based HIV incidence estimation.
Figures


Similar articles
-
A comprehensive evaluation of the proficiency testing program for the HIV-1 BED incidence assay.J Clin Microbiol. 2011 Oct;49(10):3470-3. doi: 10.1128/JCM.01122-11. Epub 2011 Aug 10. J Clin Microbiol. 2011. PMID: 21832016 Free PMC article.
-
Impact of proficiency testing program for laboratories conducting early diagnosis of HIV-1 infection in infants in low- to middle-income countries.J Clin Microbiol. 2014 Mar;52(3):773-80. doi: 10.1128/JCM.03097-13. Epub 2013 Dec 18. J Clin Microbiol. 2014. PMID: 24353004 Free PMC article.
-
Development of an international external quality assurance program for HIV-1 incidence using the Limiting Antigen Avidity assay.PLoS One. 2019 Sep 16;14(9):e0222290. doi: 10.1371/journal.pone.0222290. eCollection 2019. PLoS One. 2019. PMID: 31525218 Free PMC article.
-
Implementation of Good Clinical Laboratory Practice (GCLP) guidelines within the External Quality Assurance Program Oversight Laboratory (EQAPOL).J Immunol Methods. 2014 Jul;409:91-8. doi: 10.1016/j.jim.2013.09.012. Epub 2013 Oct 9. J Immunol Methods. 2014. PMID: 24120573 Free PMC article. Review.
-
Quality Indicators for the Total Testing Process.Clin Lab Med. 2017 Mar;37(1):187-205. doi: 10.1016/j.cll.2016.09.015. Epub 2016 Dec 10. Clin Lab Med. 2017. PMID: 28153366 Review.
Cited by
-
Anti-HIV-1 antibodies based confirmatory results in Wuhan, China, 2012-2018.PLoS One. 2020 Sep 11;15(9):e0238282. doi: 10.1371/journal.pone.0238282. eCollection 2020. PLoS One. 2020. PMID: 32915788 Free PMC article.
-
Predictors of HIV virological failure and drug resistance in Chinese patients after 48 months of antiretroviral treatment, 2008-2012: a prospective cohort study.BMJ Open. 2017 Sep 7;7(9):e016012. doi: 10.1136/bmjopen-2017-016012. BMJ Open. 2017. PMID: 28882911 Free PMC article.
-
The first 90: Progress in HIV detection in Zhejiang Province, 2008-2018.PLoS One. 2021 Apr 8;16(4):e0249517. doi: 10.1371/journal.pone.0249517. eCollection 2021. PLoS One. 2021. PMID: 33831067 Free PMC article.
-
Disparity of human immunodeficiency virus incidence and drug resistance in college student, non-student youth and older men who have sex with men: a cross-sectional study from seven major cities of China.Chin Med J (Engl). 2020 Dec 5;133(23):2778-2786. doi: 10.1097/CM9.0000000000001161. Chin Med J (Engl). 2020. PMID: 33273325 Free PMC article.
-
High HIV incidence epidemic among men who have sex with men in china: results from a multi-site cross-sectional study.Infect Dis Poverty. 2016 Sep 5;5(1):82. doi: 10.1186/s40249-016-0178-x. Infect Dis Poverty. 2016. PMID: 27593703 Free PMC article.
References
-
- Jiang Y. et al. HIV-1 incidence estimates using IgG-capture BED-enzyme immunoassay from surveillance sites of injection drug users in three cities of China. AIDS. 21, S47–S51 (2007). - PubMed
-
- Brookmeyer R. & Quinn T. C. Estimation of current human immunodeficiency virus incidence rates from a cross-sectional survey using early diagnostic tests. Am. J. Epidemiol. 141, 166–172 (1995). - PubMed
-
- McDougal J. S. et al. Surveillance for HIV-1 incidence using tests for recent infection in resource-constrained countries. AIDS. 19, S25–S30 (2005). - PubMed
-
- Murphy G. & Parry J. V. Assays for the detection of recent infections with human immunodeficiency virus type 1. Euro. Surveill. 13, 4–10 (2008). - PubMed
-
- Parekh B. S. et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res. Hum. Retroviruses. 18, 295–307 (2002). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

