Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury
- PMID: 24676640
- PMCID: PMC4073432
- DOI: 10.1681/ASN.2013070763
Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury
Abstract
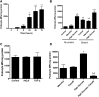
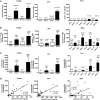
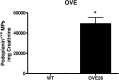
Microparticles (MPs) are small (0.1-1.0 µm) vesicles shed from the surface of cells in response to stress. Whether podocytes produce MPs and whether this production reflects glomerular injury are unclear. We examined MP formation in cultured human podocytes (hPODs) and diabetic mice. hPODs were exposed to cyclical stretch, high glucose (HG; 25 mM), angiotensin II, or TGF-β. Urinary podocyte MPs were assessed in three mouse models of diabetic nephropathy: streptozotocin (STZ)-treated, OVE26, and Akita mice. Cyclic stretch and HG increased MP release as assessed by flow cytometry (P<0.01 and P<0.05, respectively, versus controls). Inhibition of Rho-kinase (ROCK) with fasudil blocked HG-induced podocyte MP formation. STZ-treated (8 weeks) mice exhibited increased urinary podocyte MPs compared with age-matched nondiabetic mice. Similarly, 16-week-old OVE26 mice had elevated levels of urinary podocyte MPs compared with wild-type littermates (P<0.01). In 1 week post-STZ-treated and 6- and 12-week-old Akita mice, urinary podocyte MPs increased significantly compared with those MPs in nondiabetic mice, despite normal urinary albumin levels. Our results indicate that podocytes produce MPs that are released into urine. Podocyte-derived MPs are generated by exposure to mechanical stretch and high glucose in vitro and could represent early markers of glomerular injury in diabetic nephropathy.
Copyright © 2014 by the American Society of Nephrology.
Figures

Comment in
-
Tell-tale signs of perturbed podocytes.J Am Soc Nephrol. 2014 Jul;25(7):1367-9. doi: 10.1681/ASN.2014020200. Epub 2014 Mar 27. J Am Soc Nephrol. 2014. PMID: 24676633 Free PMC article. No abstract available.
References
-
- Michaud JL, Kennedy CR: The podocyte in health and disease: Insights from the mouse. Clin Sci (Lond) 112: 325–335, 2007 - PubMed
-
- Meyer TW, Bennett PH, Nelson RG: Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42: 1341–1344, 1999 - PubMed
-
- Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada N, Ebihara I, Koide H: Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol Dial Transplant 15: 1379–1383, 2000 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

