Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model
- PMID: 24677054
- PMCID: PMC4129456
- DOI: 10.1002/jcp.24636
Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model
Abstract
Estrogen deficiency is a major risk factor for osteoporosis that is associated with bone inflammation and resorption. Half of women over the age of 50 will experience an osteoporosis related fracture in their lifetime, thus novel therapies are needed to combat post-menopausal bone loss. Recent studies suggest an important role for gut-bone signaling pathways and the microbiota in regulating bone health. Given that the bacterium Lactobacillus reuteri ATCC PTA 6475 (L. reuteri) secretes beneficial immunomodulatory factors, we examined if this candidate probiotic could reduce bone loss associated with estrogen deficiency in an ovariectomized (Ovx) mouse menopausal model. Strikingly, L. reuteri treatment significantly protected Ovx mice from bone loss. Osteoclast bone resorption markers and activators (Trap5 and RANKL) as well as osteoclastogenesis are significantly decreased in L. reuteri-treated mice. Consistent with this, L. reuteri suppressed Ovx-induced increases in bone marrow CD4+ T-lymphocytes (which promote osteoclastogenesis) and directly suppressed osteoclastogenesis in vitro. We also identified that L. reuteri treatment modifies microbial communities in the Ovx mouse gut. Together, our studies demonstrate that L. reuteri treatment suppresses bone resorption and loss associated with estrogen deficiency. Thus, L. reuteri treatment may be a straightforward and cost-effective approach to reduce post-menopausal bone loss.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
All authors state that they have no conflict of interest.
Figures



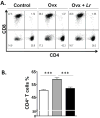

References
-
- ESHRE Capri Workshop Group. Bone fractures after menopause. Hum Reprod Update. 2010;16(6):761–773. - PubMed
-
- Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, Ellis KJ. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. The American journal of clinical nutrition. 2005;82(2):471–476. - PubMed
-
- Backhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, Versalovic J, Young V, Finlay BB. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell host & microbe. 2012;12(5):611–622. - PubMed
-
- Bouxsein ML, Myers KS, Shultz KL, Donahue LR, Rosen CJ, Beamer WG. Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res. 2005;20(7):1085–1092. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

