Retinal pigment epithelial cell death by the alternative complement cascade: role of membrane regulatory proteins, calcium, PKC, and oxidative stress
- PMID: 24677108
- PMCID: PMC4581691
- DOI: 10.1167/iovs.13-13554
Retinal pigment epithelial cell death by the alternative complement cascade: role of membrane regulatory proteins, calcium, PKC, and oxidative stress
Abstract
Purpose: Retinal pigment epithelial (RPE) cell death is an important feature of the advanced forms of AMD. Complement alternative pathway (AP) activation is associated with RPE cell death in AMD. In this study, we developed a new model to initiate AP activation on RPE cells and investigated the cellular mechanisms modulating AP activation-mediated RPE cell death.
Methods: An anti-RPE antibody was developed. A spontaneously arising human RPE cell line (ARPE-19) and donor RPE cells were primed with this antibody followed by stimulation with 6% C1q-depleted human serum (C1q-Dep) to activate AP. Complement activation was evaluated by flow cytometry and immunofluorescent staining. Cellular response to complement activation was examined by measurement of intracellular calcium and adenosine triphosphate (ATP) release. Cell viability was assessed by Sytox orange, tetrazolium salt, and lactate dehydrogenase release assays.
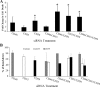
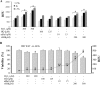
Results: Alternative pathway complement-mediated RPE cell death was associated with membrane attack complex formation and a rapid rise in intracellular calcium followed by release of ATP. Downregulation of membrane complement regulatory proteins and protein kinase C (PKC) inhibition increased cell susceptibility to complement attack. Pretreatment of RPE cells with either hydrogen peroxide or hydroquinone enhanced cell death. Chronic repetitive treatment of RPE cells with low levels of oxidants also enhanced complement-mediated cell death.
Conclusions: Activation of complement through the alternative pathway induces sublytic and lytic phases of complement attack on RPE cells, leading to cell death modulated by extracellular calcium, membrane complement regulatory proteins, and intracellular signaling mechanisms. Single-dose oxidant exposure and low-dose repetitive oxidant exposure rendered RPE cells more susceptible to complement-mediated death.
Keywords: AMD; PKC; RPE; calcium; complement; oxidative stress.
Figures





References
-
- Johnson LV, Leitner WP, Staples MK, Anderson DH. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Exp Eye Res. 2001; 73: 887– 896. - PubMed
-
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000; 14: 835– 846. - PubMed
-
- Edwards AO, Ritter R III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005; 308: 421– 424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

