Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals
- PMID: 24677184
- PMCID: PMC4077973
- DOI: 10.1002/hep.27151
Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 hepatitis C virus in interferon-ineligible/intolerant individuals
Abstract
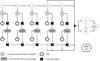
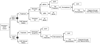
Treatment guidance for chronic hepatitis C (CHC) released by the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) offers two options for interferon (IFN)-ineligible/intolerant individuals with genotype 1 infection: sofosbuvir/ribavirin (SOF/RBV) for 24 weeks or sofosbuvir/simeprevir (SOF/SMV) for 12 weeks. A 24-week course of SOF/RBV costs approximately US$169,000, with sustained virologic response (SVR) rates ranging from 52% to 84%; 12 weeks of SOF/SMV costs approximately $150,000, with SVR between 89% and 100%. Because SOF/SMV is currently used off-label, debate exists among physicians and payers about whether it should be prescribed and covered. This article presents a cost-effectiveness analysis of these two treatment regimens accounting for costs of drugs, treatment-related medical care, retreatment for individuals who do not achieve SVR, and natural history of continued HCV infection after failed retreatment. Analysis uses a Markov model with a lifetime horizon and a societal perspective. In the base-case scenario, SOF/SMV dominated SOF/RBV in a modeled 50-year-old cohort of treatment-naïve and -experienced subjects, excluding those who failed earlier therapy with telaprevir or boceprevir. SOF/SMV yielded lower costs and more quality-adjusted life years (QALYs) for the average subject, compared to SOF/RBV ($165,336 and 14.69 QALYs vs. $243,586 and 14.45 QALYs, respectively). In base-case cost analysis, the SOF/SMV treatment strategy saved $91,590 per SVR, compared to SOF/RBV. Under all one-way sensitivity scenarios, SOF/SMV remained dominant and resulted in cost savings.
Conclusions: These results suggest that a 12-week course of SOF/SMV is a more cost-effective treatment for genotype 1 CHC than 24 weeks of SOF/RBV among IFN-ineligible/intolerant individuals, supporting the AASLD/IDSA guidance and offering implications for both clinical and regulatory decision making as well as pharmaceutical pricing.
© 2014 by the American Association for the Study of Liver Diseases. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Figures
Comment in
-
Valuing cure: bridging cost-effectiveness and coverage decisions for hepatitis C therapy.Hepatology. 2014 Jul;60(1):12-4. doi: 10.1002/hep.27220. Hepatology. 2014. PMID: 24825115 Free PMC article. No abstract available.
-
The good, the bad and the ugly of the new treatments for hepatitis C virus.Ann Hepatol. 2014 Sep-Oct;13(5):574-5. Ann Hepatol. 2014. PMID: 25152995 No abstract available.
References
-
- American Association for the Study of Liver Diseases and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. 2014 Retrieved from: http://www.hcvguidelines.org/full-report-view. - PMC - PubMed
-
- US Food and Drug Administration. OLYSIO. 2013 Retrieved from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/205123s001lbl.pdf.
-
- US Food and Drug Administration. SOVALDI. 2013 Retrieved from: http://www.accessdata.fda.gov/spl/data/b0de1fcd-6d03-4a91-a7df-72a14c8bc....
-
- Bini EJ, Brau N, Currie S, Shen H, Anand BS, Hu KQ, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C virus infection. Am J Gastroenterol. 2005;100:1772–1779. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
